The embryonic type of SPP1 transcriptional regulation is re-activated in glioblastoma
- PMID: 28030801
- PMCID: PMC5369967
- DOI: 10.18632/oncotarget.14092
The embryonic type of SPP1 transcriptional regulation is re-activated in glioblastoma
Abstract
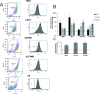
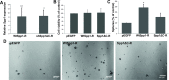
Osteopontin (SPP1, a secreted phosphoprotein 1) is primarily involved in immune responses, tissue remodelling and biomineralization. However, it is also overexpressed in many cancers and regulates tumour progression by increasing migration, invasion and cancer stem cell self-renewal. Mechanisms of SPP1 overexpression in gliomas are poorly understood. We demonstrate overexpression of two out of five SPP1 isoforms in glioblastoma (GBM) and differential isoform expression in glioma cell lines. Up-regulated SPP1 expression is associated with binding of the GLI1 transcription factor to the promoter and OCT4 (octamer-binding transcription factor 4) to the first SPP1 intron of the SPP1 gene in human glioma cells but not in non-transformed astrocytes. GLI1 knockdown reduced SPP1 mRNA and protein levels in glioma cells. GLI1 and OCT4 are known regulators of stem cell pluripotency. GBMs contain rare cells that express stem cell markers and display ability to self-renew. We reveal that SPP1 is overexpressed in glioma initiating cells defined by high rhodamine 123 efflux, sphere forming capacity and stemness marker expression. Forced differentiation of human glioma spheres reduced SPP1 expression. Knockdown of SPP1, GLI1 or CD44 with siRNAs diminished sphere formation. C6 glioma cells stably depleted of Spp1 displayed reduced sphere forming capacity and downregulated stemness marker expression. Overexpression of the wild type Spp1, but not Spp1 lacking a Cd44 binding domain, rescued cell ability to form spheres. Our findings show re-activation of the embryonic-type transcriptional regulation of SPP1 in malignant gliomas and point to the importance of SPP1-CD44 interactions in self-renewal and pluripotency glioma initiating cells.
Keywords: glioma initiating cells; osteopontin; self-renewal; stemness factors; transcription factors.
Conflict of interest statement
None.
Figures





References
-
- Tuck AB, Chambers AF, Allan AL. Osteopontin overexpression in breast cancer: knowledge gained and possible implications for clinical management. J Cell Biochem. 2007;102:859–868. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

