Drug-induced premature senescence model in human dental follicle stem cells
- PMID: 28030852
- PMCID: PMC5352320
- DOI: 10.18632/oncotarget.14085
Drug-induced premature senescence model in human dental follicle stem cells
Abstract
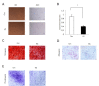
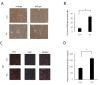
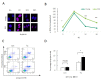
Aging is identified by a progressive decline of physiological integrity leading to age-related degenerative diseases, but its causes is unclear. Human dental pulp stem cells (hDPSCs) has a remarkable rejuvenated capacity that relies on its resident stem cells. However, because of the lack of proper senescence models, exploration of the underlying molecular mechanisms has been hindered. Here, we established a cellular model utilizing a hydroxyurea (HU) treatment protocol and effectively induced Human dental pulp stem cells to undergo cellular senescence. Age-related phenotypic changes were identified by augmented senescence-associated-β-galactosidase (SA-β-gal) staining, declined proliferation and differentiation capacity, elevated G0/G1 cell cycle arrest, increased apoptosis and reactive oxygen species levels. Furthermore, we tested the expression of key genes in various DNA repair pathways including nonhomologous end-joining (NHEJ) and homologous recombination (HR) pathways. In addition, our results showed that Dental pulp stem cells from young donors are more resistant to apoptosis and exhibit increased non-homologous end joining activity compared to old donors. Further transcriptome analysis demonstrate that multiple pathways are involved in the HU-induced Dental pulp stem cells ageing, including genes associated with DNA damage and repair, mitochondrial dysfunction and increased reactive oxygen species levels. Taken together, the cellular model have important implications for understanding the molecular exploration of Dental pulp stem cells senescence and aging.
Keywords: DNA damage; Gerotarget; aging; cellular senescence model; dental stem cells; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120(4):437–447. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

