Imaging of Intracranial Hemorrhage
- PMID: 28030895
- PMCID: PMC5307932
- DOI: 10.5853/jos.2016.00563
Imaging of Intracranial Hemorrhage
Abstract
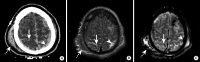
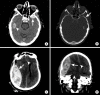
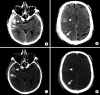
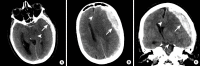
Intracranial hemorrhage is common and is caused by diverse pathology, including trauma, hypertension, cerebral amyloid angiopathy, hemorrhagic conversion of ischemic infarction, cerebral aneurysms, cerebral arteriovenous malformations, dural arteriovenous fistula, vasculitis, and venous sinus thrombosis, among other causes. Neuroimaging is essential for the treating physician to identify the cause of hemorrhage and to understand the location and severity of hemorrhage, the risk of impending cerebral injury, and to guide often emergent patient treatment. We review CT and MRI evaluation of intracranial hemorrhage with the goal of providing a broad overview of the diverse causes and varied appearances of intracranial hemorrhage.
Keywords: Epidural hematoma; Head trauma; Intracranial hemorrhage; Intraparenchymal hemorrhage; Subarachnoid hemorrhage; Subdural hematoma.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
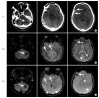
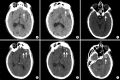
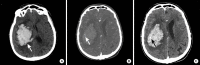
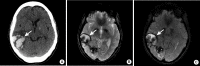
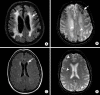
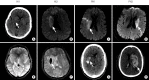
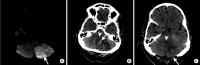
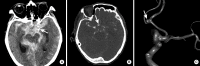



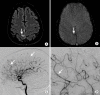
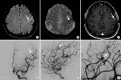
References
-
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. - PubMed
-
- Shetty VS, Reis MN, Aulino JM, Berger KL, Broder J, Choudhri AF, et al. ACR appropriateness criteria head trauma. J Am Coll Radiol. 2016;13:668–679. - PubMed
-
- Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma. 2008;25:1049–1056. - PubMed
-
- Altmeyer W, Steven A, Gutierrez J. Use of magnetic resonance in the evaluation of cranial trauma. Magn Reson Imaging Clin N Am. 2016;24:305–323. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

