Metabolic model of central carbon and energy metabolisms of growing Arabidopsis thaliana in relation to sucrose translocation
- PMID: 28031032
- PMCID: PMC5192601
- DOI: 10.1186/s12870-016-0868-3
Metabolic model of central carbon and energy metabolisms of growing Arabidopsis thaliana in relation to sucrose translocation
Abstract
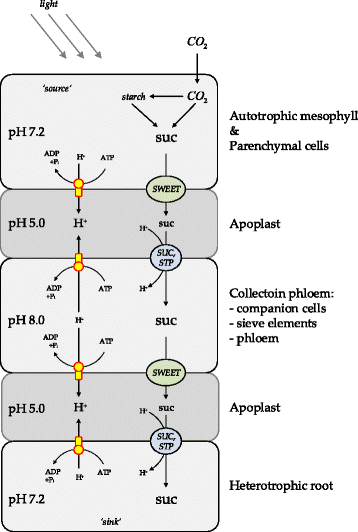
Background: Sucrose translocation between plant tissues is crucial for growth, development and reproduction of plants. Systemic analysis of these metabolic and underlying regulatory processes allow a detailed understanding of carbon distribution within the plant and the formation of associated phenotypic traits. Sucrose translocation from 'source' tissues (e.g. mesophyll) to 'sink' tissues (e.g. root) is tightly bound to the proton gradient across the membranes. The plant sucrose transporters are grouped into efflux exporters (SWEET family) and proton-symport importers (SUC, STP families). To better understand regulation of sucrose export from source tissues and sucrose import into sink tissues, there is a need for a metabolic model that takes in account the tissue organisation of Arabidopsis thaliana with corresponding metabolic specificities of respective tissues in terms of sucrose and proton production/utilization. An ability of the model to operate under different light modes ('light' and 'dark') and correspondingly in different energy producing modes is particularly important in understanding regulatory modules.
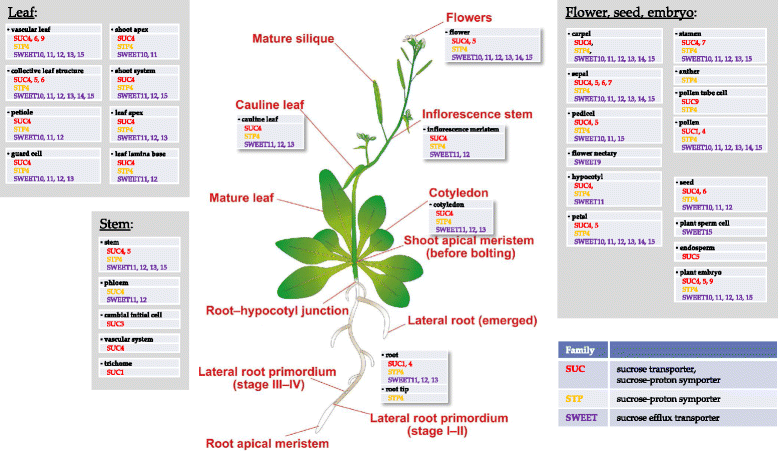
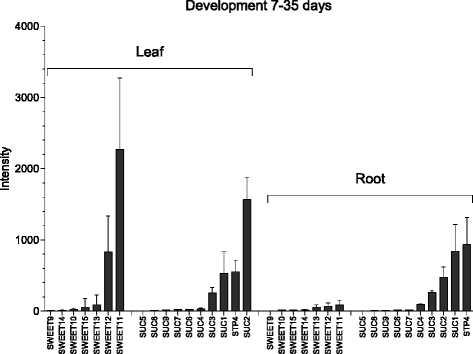
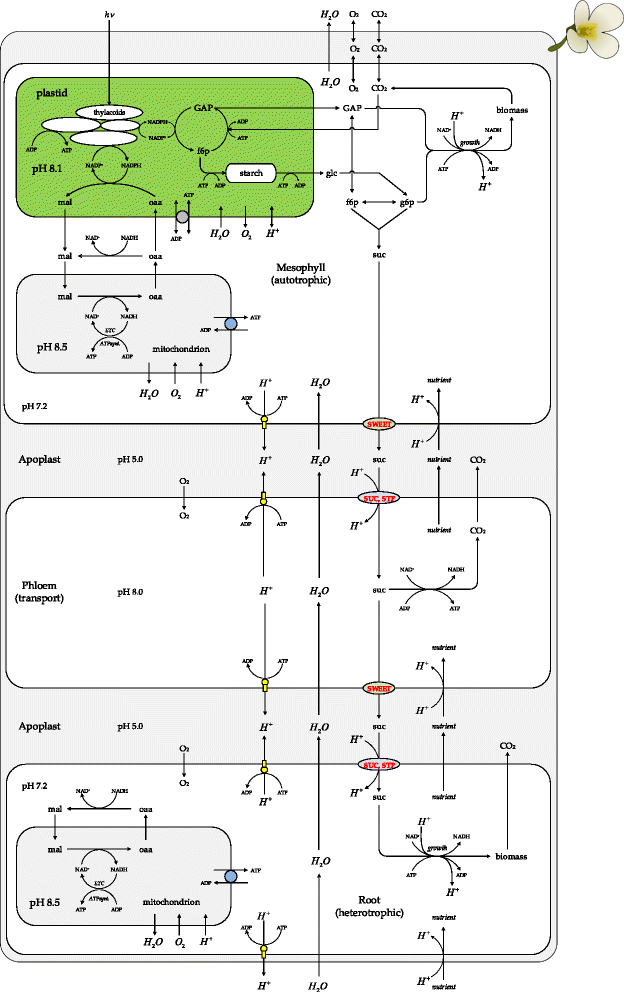
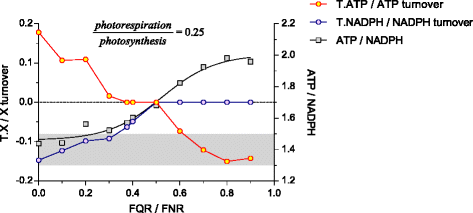
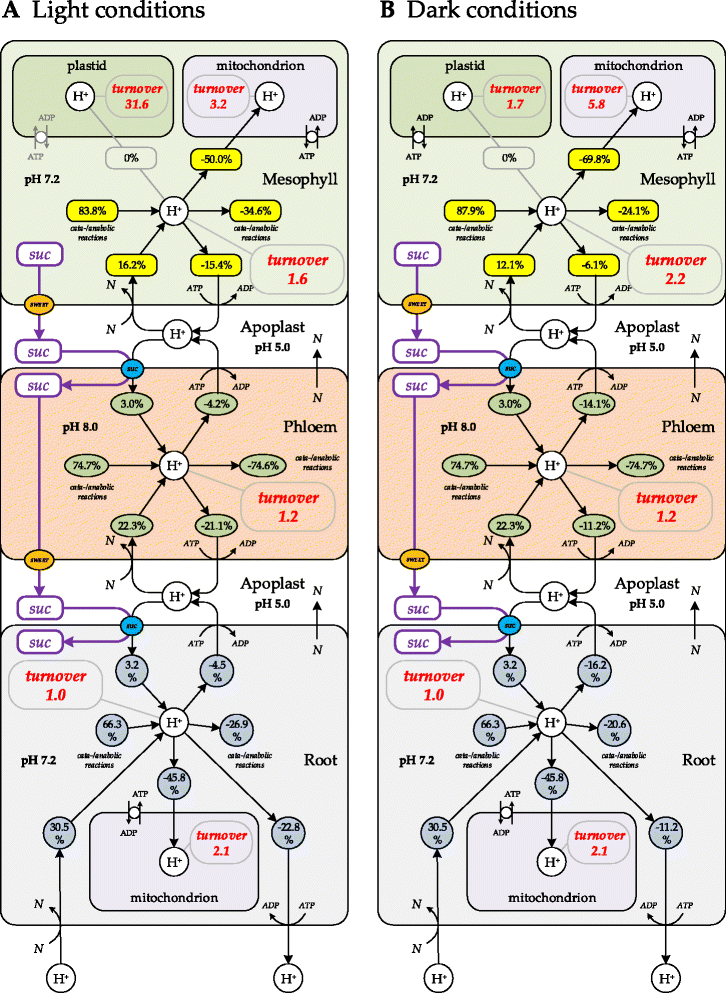
Results: Here, we describe a multi-compartmental model consisting of a mesophyll cell with plastid and mitochondrion, a phloem cell, as well as a root cell with mitochondrion. In this model, the phloem was considered as a non-growing transport compartment, the mesophyll compartment was considered as both autotrophic (growing on CO2 under light) and heterotrophic (growing on starch in darkness), and the root was always considered as heterotrophic tissue dependent on sucrose supply from the mesophyll compartment. In total, the model includes 413 balanced compounds interconnected by 400 transformers. The structured metabolic model accounts for central carbon metabolism, photosynthesis, photorespiration, carbohydrate metabolism, energy and redox metabolisms, proton metabolism, biomass growth, nutrients uptake, proton gradient generation and sucrose translocation between tissues. Biochemical processes in the model were associated with gene-products (742 ORFs). Flux Balance Analysis (FBA) of the model resulted in balanced carbon, nitrogen, proton, energy and redox states under both light and dark conditions. The main H+-fluxes were reconstructed and their directions matched with proton-dependent sucrose translocation from 'source' to 'sink' under any light condition.
Conclusions: The model quantified the translocation of sucrose between plant tissues in association with an integral balance of protons, which in turn is defined by operational modes of the energy metabolism.
Keywords: Central carbon metabolism; Diurnal growth; Energy metabolism; Flux balance analysis; Multi-compartment metabolic model; Sucrose metabolism; Sucrose transport.
Figures

Similar articles
-
Using a Multi-compartmental Metabolic Model to Predict Carbon Allocation in Arabidopsis thaliana.Methods Mol Biol. 2019;2014:345-369. doi: 10.1007/978-1-4939-9562-2_27. Methods Mol Biol. 2019. PMID: 31197808
-
Constitutive and Companion Cell-Specific Overexpression of AVP1, Encoding a Proton-Pumping Pyrophosphatase, Enhances Biomass Accumulation, Phloem Loading, and Long-Distance Transport.Plant Physiol. 2016 Jan;170(1):401-14. doi: 10.1104/pp.15.01409. Epub 2015 Nov 3. Plant Physiol. 2016. PMID: 26530315 Free PMC article.
-
Effective carbon partitioning driven by exotic phloem-specific regulatory elements fused to the Arabidopsis thaliana AtSUC2 sucrose-proton symporter gene.BMC Plant Biol. 2009 Jan 20;9:7. doi: 10.1186/1471-2229-9-7. BMC Plant Biol. 2009. PMID: 19154603 Free PMC article.
-
Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security.J Exp Bot. 2014 Apr;65(7):1713-35. doi: 10.1093/jxb/ert416. Epub 2013 Dec 17. J Exp Bot. 2014. PMID: 24347463 Review.
-
Sugars en route to the roots. Transport, metabolism and storage within plant roots and towards microorganisms of the rhizosphere.Physiol Plant. 2019 Jan;165(1):44-57. doi: 10.1111/ppl.12751. Epub 2018 Sep 3. Physiol Plant. 2019. PMID: 29704246 Review.
Cited by
-
Oxaloacetate induces apoptosis in HepG2 cells via inhibition of glycolysis.Cancer Med. 2018 Apr;7(4):1416-1429. doi: 10.1002/cam4.1410. Epub 2018 Mar 13. Cancer Med. 2018. PMID: 29533007 Free PMC article.
-
Flux balance analysis of cyanobacteria reveals selective use of photosynthetic electron transport components under different spectral light conditions.Photosynth Res. 2020 Jan;143(1):31-43. doi: 10.1007/s11120-019-00678-x. Epub 2019 Oct 17. Photosynth Res. 2020. PMID: 31625072
-
A genome-scale TF-DNA interaction network of transcriptional regulation of Arabidopsis primary and specialized metabolism.Mol Syst Biol. 2021 Nov;17(11):e10625. doi: 10.15252/msb.202110625. Mol Syst Biol. 2021. PMID: 34816587 Free PMC article.
-
A Holistic Approach to Study Photosynthetic Acclimation Responses of Plants to Fluctuating Light.Front Plant Sci. 2021 Apr 14;12:668512. doi: 10.3389/fpls.2021.668512. eCollection 2021. Front Plant Sci. 2021. PMID: 33936157 Free PMC article. Review.
-
In planta study of photosynthesis and photorespiration using NADPH and NADH/NAD+ fluorescent protein sensors.Nat Commun. 2020 Jun 26;11(1):3238. doi: 10.1038/s41467-020-17056-0. Nat Commun. 2020. PMID: 32591540 Free PMC article.
References
-
- Taiz L, Zeiger E. Plant physiology, 5th edn. Sunderland: Sinauer Associates, Inc.; 2010.
-
- Chapin FS, Schulze ED, Mooney HA. The ecology and economics of storage in plants. Annu Rev Ecol Syst. 1990;21:423–47. doi: 10.1146/annurev.es.21.110190.002231. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

