PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR
- PMID: 28031179
- PMCID: PMC5391777
- DOI: 10.1182/blood-2016-09-738245
PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR
Figures
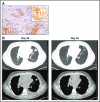
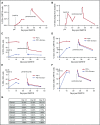
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

