Molecular magnetic resonance imaging discloses endothelial activation after transient ischaemic attack
- PMID: 28031221
- PMCID: PMC5226059
- DOI: 10.1093/brain/aww260
Molecular magnetic resonance imaging discloses endothelial activation after transient ischaemic attack
Abstract
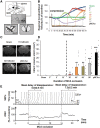
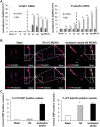
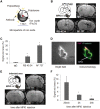
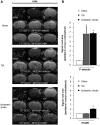
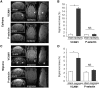
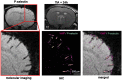
SEE SUN ET AL DOI101093/AWW306 FOR A SCIENTIFIC COMMENTARY ON THIS ARTICLE: About 20% of patients with ischaemic stroke have a preceding transient ischaemic attack, which is clinically defined as focal neurological symptoms of ischaemic origin resolving spontaneously. Failure to diagnose transient ischaemic attack is a wasted opportunity to prevent recurrent disabling stroke. Unfortunately, diagnosis can be difficult, due to numerous mimics, and to the absence of a specific test. New diagnostic tools are thus needed, in particular for radiologically silent cases, which correspond to the recommended tissue-based definition of transient ischaemic attack. As endothelial activation is a hallmark of cerebrovascular events, we postulated that this may also be true for transient ischaemic attack, and that it would be clinically relevant to develop non-invasive in vivo imaging to detect this endothelial activation. Using transcriptional and immunohistological analyses for adhesion molecules in a mouse model, we identified brain endothelial P-selectin as a potential biomarker for transient ischaemic attack. We thus developed ultra-sensitive molecular magnetic resonance imaging using antibody-based microparticles of iron oxide targeting P-selectin. This highly sensitive imaging strategy unmasked activated endothelial cells after experimental transient ischaemic attack and allowed discriminating transient ischaemic attack from epilepsy and migraine, two important transient ischaemic attack mimics. We provide preclinical evidence that combining conventional magnetic resonance imaging with molecular magnetic resonance imaging targeting P-selectin might aid in the diagnosis of transient ischaemic attack.
Keywords: P-selectin; cerebrovascular inflammation; mimics; molecular imaging; transient ischaemic attack.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
Fleeting footprints: finding MRI biomarkers of transient ischaemic attack.Brain. 2017 Jan;140(1):8-10. doi: 10.1093/brain/aww306. Brain. 2017. PMID: 28031217 No abstract available.
References
-
- Arsava EM, Gurer G, Gursoy-Ozdemir Y, Karatas H, Dalkara T. A new model of transient focal cerebral ischemia for inducing selective neuronal necrosis. Brain Res Bull 2009; 78: 226–31. - PubMed
-
- Battistini L, Piccio L, Rossi B, Bach S, Galgani S, Gasperini C, et al.CD8+ T cells from patients with acute multiple sclerosis display selective increase of adhesiveness in brain venules: a critical role for P-selectin glycoprotein ligand-1. Blood 2003; 101: 4775–82. - PubMed
-
- Berti R, Williams AJ, Moffett JR, Hale SL, Velarde LC, Elliott PJ, et al.Quantitative real-time RT-PCR analysis of inflammatory gene expression associated with ischemia-reperfusion brain injury. J Cereb Blood Flow Metab 2002; 22: 1068–79. - PubMed
-
- Beziere N, von Schacky C, Kosanke Y, Kimm M, Nunes A, Licha K, et al.Optoacoustic imaging and staging of inflammation in a murine model of arthritis. Arthritis Rheumatol 2014; 66: 2071–8. - PubMed
-
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al.Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40: 2276–93. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

