Implementing medical abortion with mifepristone and misoprostol in Norway 1998-2013
- PMID: 28031316
- PMCID: PMC5837406
- DOI: 10.1093/ije/dyw270
Implementing medical abortion with mifepristone and misoprostol in Norway 1998-2013
Abstract
Background: Medical abortion with mifepristone and misoprostol was introduced in Norway in 1998, and since then there has been an almost complete change from predominantly surgical to medical abortions. We aimed to describe the medical abortion implementation process, and to compare characteristics of women obtaining medical and surgical abortion.
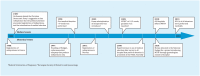
Methods: Information from all departments of obstetrics and gynaecology in Norway on the time of implementation of medical abortion and abortion procedures in use up to 12 weeks of gestation was assessed by surveys in 2008 and 2012. We also analysed data from the National Abortion Registry comprising 223 692 women requesting abortion up to 12 weeks of gestation during 1998-2013.
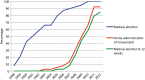
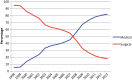
Results: In 2012, all hospitals offered medical abortion, 84.4% offered medical abortion at 9-12 weeks of gestation and 92.1% offered home administration of misoprostol. The use of medical abortion increased from 5.9% of all abortions in 1998 to 82.1% in 2013. Compared with women having a surgical abortion, women obtaining medical abortion had higher odds for undergoing an abortion at 4-6 weeks (adjusted OR 2.33; 95% confidence interval 2.28-2.38). Waiting time between registered request for an abortion until termination was reduced from 11.3 days in 1998 to 7.3 days in 2013.
Conclusions: Norwegian women have gained access to more treatment modalities and simplified protocols for medical abortion. At the same time they obtained abortions at an earlier gestational age and the waiting time has been reduced.
Keywords: Medical abortion; Norway; mifepristone; misoprostol; registry.
© The Author 2016. Published by Oxford University Press on behalf of the International Epidemiological Association
Figures
References
-
- Grimes DA, Benson J, Singh S. et al. Unsafe abortion: the preventable pandemic. Lancet 2006;368(9550):1908–19. - PubMed
-
- Sedgh G, Singh S, Shah IH, Åhman E, Henshaw SK, Bankole A.. Induced abortion: incidence and trends worldwide from 1995 to 2008. Lancet 2012;379(9816):625–32. - PubMed
-
- Løkeland M, Akerkar R, Askeland OM. et al. Rapport om svangerskapsavbrudd for 2015. [Termination of pregnancy report for 2015] 2016. http://www.fhi.no/dokumenter/92e78ccc42.pdf (19 April 2016, date last accessed).
-
- Norwegian Parliament St.meld nr 16 (1995-96) Om erfaringer med lov om svangerskapsavbrudd mv. [White Paper number 16 (1995-96) On experiences with the termination of pregnancy law] Oslo: Ministry of Health, 1997.
-
- The Norwegian Medicines Agency Mifegyne - 200 mg 2014. http://www.legemiddelverket.no/Legemiddelsoek/Sider/Legemiddelvisning.as... (12 December 2014, date last accessed).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical