INPP5E Preserves Genomic Stability through Regulation of Mitosis
- PMID: 28031327
- PMCID: PMC5335510
- DOI: 10.1128/MCB.00500-16
INPP5E Preserves Genomic Stability through Regulation of Mitosis
Erratum in
-
Correction for Sierra Potchanant et al., "INPP5E Preserves Genomic Stability through Regulation of Mitosis".Mol Cell Biol. 2017 Sep 26;37(20):e00389-17. doi: 10.1128/MCB.00389-17. Print 2017 Oct 15. Mol Cell Biol. 2017. PMID: 28951484 Free PMC article. No abstract available.
Abstract
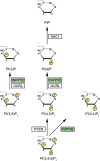
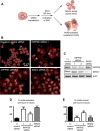
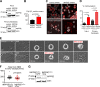
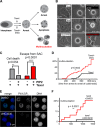
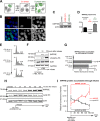
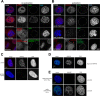
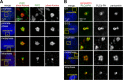
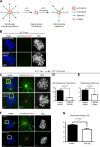
The partially understood phosphoinositide signaling cascade regulates multiple aspects of cellular metabolism. Previous studies revealed that INPP5E, the inositol polyphosphate-5-phosphatase that is mutated in the developmental disorders Joubert and MORM syndromes, is essential for the function of the primary cilium and maintenance of phosphoinositide balance in nondividing cells. Here, we report that INPP5E further contributes to cellular homeostasis by regulating cell division. We found that silencing or genetic knockout of INPP5E in human and murine cells impairs the spindle assembly checkpoint, centrosome and spindle function, and maintenance of chromosomal integrity. Consistent with a cell cycle regulatory role, we found that INPP5E expression is cell cycle dependent, peaking at mitotic entry. INPP5E localizes to centrosomes, chromosomes, and kinetochores in early mitosis and shuttles to the midzone spindle at mitotic exit. Our findings identify the previously unknown, essential role of INPP5E in mitosis and prevention of aneuploidy, providing a new perspective on the function of this phosphoinositide phosphatase in health and development.
Keywords: INPP5E; aneuploidy; cell cycle; centrosomes; mitosis; spindle assembly checkpoint.
Copyright © 2017 Sierra Potchanant et al.
Figures




References
-
- Maffucci T. 2012. An introduction to phosphoinositides, p 1–42. In Falasca M. (ed), Phosphoinositides and disease. Springer Science+Business Media, Dordrecht, the Netherlands.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
