Phosphoribosyl Diphosphate (PRPP): Biosynthesis, Enzymology, Utilization, and Metabolic Significance
- PMID: 28031352
- PMCID: PMC5312242
- DOI: 10.1128/MMBR.00040-16
Phosphoribosyl Diphosphate (PRPP): Biosynthesis, Enzymology, Utilization, and Metabolic Significance
Abstract
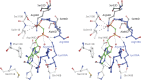
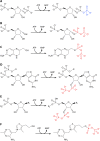
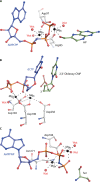
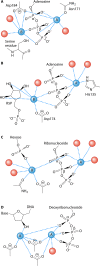
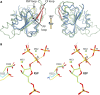
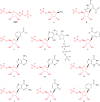
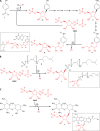
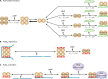
Phosphoribosyl diphosphate (PRPP) is an important intermediate in cellular metabolism. PRPP is synthesized by PRPP synthase, as follows: ribose 5-phosphate + ATP → PRPP + AMP. PRPP is ubiquitously found in living organisms and is used in substitution reactions with the formation of glycosidic bonds. PRPP is utilized in the biosynthesis of purine and pyrimidine nucleotides, the amino acids histidine and tryptophan, the cofactors NAD and tetrahydromethanopterin, arabinosyl monophosphodecaprenol, and certain aminoglycoside antibiotics. The participation of PRPP in each of these metabolic pathways is reviewed. Central to the metabolism of PRPP is PRPP synthase, which has been studied from all kingdoms of life by classical mechanistic procedures. The results of these analyses are unified with recent progress in molecular enzymology and the elucidation of the three-dimensional structures of PRPP synthases from eubacteria, archaea, and humans. The structures and mechanisms of catalysis of the five diphosphoryltransferases are compared, as are those of selected enzymes of diphosphoryl transfer, phosphoryl transfer, and nucleotidyl transfer reactions. PRPP is used as a substrate by a large number phosphoribosyltransferases. The protein structures and reaction mechanisms of these phosphoribosyltransferases vary and demonstrate the versatility of PRPP as an intermediate in cellular physiology. PRPP synthases appear to have originated from a phosphoribosyltransferase during evolution, as demonstrated by phylogenetic analysis. PRPP, furthermore, is an effector molecule of purine and pyrimidine nucleotide biosynthesis, either by binding to PurR or PyrR regulatory proteins or as an allosteric activator of carbamoylphosphate synthetase. Genetic analyses have disclosed a number of mutants altered in the PRPP synthase-specifying genes in humans as well as bacterial species.
Keywords: amino acid metabolism; diphosphoryl transfer; nucleotide metabolism; phosphoribosyl pyrophosphate; protein structure-function.
Copyright © 2016 American Society for Microbiology.
Figures














References
-
- Jensen KF. 1983. Metabolism of 5-phosphoribosyl 1-pyrophosphate (PRPP) in Escherichia coli and Salmonella typhimurium, p 1–25. In Munch-Petersen A. (ed), Metabolism of nucleotides, nucleosides and nucleobases in microorganisms. Academic Press, Inc., London, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

