Intra-arterial nitroglycerin as directed acute treatment in experimental ischemic stroke
- PMID: 28031354
- PMCID: PMC5489382
- DOI: 10.1136/neurintsurg-2016-012793
Intra-arterial nitroglycerin as directed acute treatment in experimental ischemic stroke
Abstract
Background: Nitroglycerin (also known as glyceryl trinitrate (GTN)), a vasodilator best known for treatment of ischemic heart disease, has also been investigated for its potential therapeutic benefit in ischemic stroke. The completed Efficacy of Nitric Oxide in Stroke trial suggested that GTN has therapeutic benefit with acute (within 6 hours) transdermal systemic sustained release therapy.
Objective: To examine an alternative use of GTN as an acute therapy for ischemic stroke following successful recanalization.
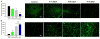
Methods: We administered GTN IA following transient middle cerebral artery occlusion in mice. Because no standard dose of GTN is available following emergent large vessel occlusion, we performed a dose-response (3.12, 6.25, 12.5, and 25 µg/µL) analysis. Next, we looked at blood perfusion (flow) through the middle cerebral artery using laser Doppler flowmetry. Functional outcomes, including forced motor movement rotor rod, were assessed in the 3.12, 6.25, and 12.5 µg/µL groups. Histological analysis was performed using cresyl violet for infarct volume, and glial fibrillary activating protein (GFAP) and NeuN immunohistochemistry for astrocyte activation and mature neuron survival, respectively.
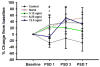
Results: Overall, we found that acute post-stroke IA GTN had little effect on vessel dilatation after 15 min. Functional analysis showed a significant difference between GTN (3.12 and 6.25 µg/µL) and control at post-stroke day 1. Histological measures showed a significant reduction in infarct volume and GFAP immunoreactivity and a significant increase in NeuN.
Conclusions: These results demonstrate that acute IA GTN is neuroprotective in experimental ischemic stroke and warrants further study as a potentially new stroke therapy.
Keywords: Blood Flow; Drug; Intracranial Pressure; Pharmacology; Stroke.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures
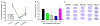

References
-
- Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–9. - PubMed
-
- Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. - PubMed
-
- Parsons MW, Albers GW. MR RESCUE: is the glass half-full or half-empty? Stroke. 2013;44:2055–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
