Binding of the Methyl Donor S-Adenosyl-l-Methionine to Middle East Respiratory Syndrome Coronavirus 2'- O-Methyltransferase nsp16 Promotes Recruitment of the Allosteric Activator nsp10
- PMID: 28031370
- PMCID: PMC5309940
- DOI: 10.1128/JVI.02217-16
Binding of the Methyl Donor S-Adenosyl-l-Methionine to Middle East Respiratory Syndrome Coronavirus 2'- O-Methyltransferase nsp16 Promotes Recruitment of the Allosteric Activator nsp10
Abstract
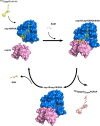
The Middle East respiratory syndrome coronavirus (MERS-CoV) nonstructural protein 16 (nsp16) is an S-adenosyl-l-methionine (SAM)-dependent 2'-O-methyltransferase (2'-O-MTase) that is thought to methylate the ribose 2'-OH of the first transcribed nucleotide (N1) of viral RNA cap structures. This 2'-O-MTase activity is regulated by nsp10. The 2'-O methylation prevents virus detection by cell innate immunity mechanisms and viral translation inhibition by the interferon-stimulated IFIT-1 protein. To unravel the regulation of nsp10/nsp16 2'-O-MTase activity, we used purified MERS-CoV nsp16 and nsp10. First, we showed that nsp16 recruited N7-methylated capped RNA and SAM. The SAM binding promotes the assembly of the enzymatically active nsp10/nsp16 complex that converted 7mGpppG (cap-0) into 7mGpppG2'Om (cap-1) RNA by 2'-OH methylation of N1 in a SAM-dependent manner. The subsequent release of SAH speeds up nsp10/nsp16 dissociation that stimulates the reaction turnover. Alanine mutagenesis and RNA binding assays allowed the identification of the nsp16 residues involved in RNA recognition forming the RNA binding groove (K46, K170, E203, D133, R38, Y47, and Y181) and the cap-0 binding site (Y30, Y132, and H174). Finally, we found that nsp10/nsp16 2'-O-MTase activity is sensitive to known MTase inhibitors, such as sinefungin and cap analogues. This characterization of the MERS-CoV 2'-O-MTase is a preliminary step toward the development of molecules to inhibit cap 2'-O methylation and to restore the host antiviral response. IMPORTANCE MERS-CoV codes for a cap 2'-O-methyltransferase that converts cap-0 into cap-1 structure in order to prevent virus detection by cell innate immunity mechanisms. We report the biochemical properties of MERS-CoV 2'O-methyltransferase, which is stimulated by nsp10 acting as an allosteric activator of the nsp16 2'-O-methyltransferase possibly through enhanced RNA binding affinity. In addition, we show that SAM promotes the formation of the active nsp10/nsp16 complex. Conversely, after cap methylation, the reaction turnover is speeded up by cap-1 RNA release and nsp10/nsp16 complex dissociation, at the low intracellular SAH concentration. These results suggest that SAM/SAH balance is a regulator of the 2'-O-methyltransferase activity and raises the possibility that SAH hydrolase inhibitors might interfere with CoV replication cycle. The enzymatic and RNA binding assays developed in this work were also used to identify nsp16 residues involved in cap-0 RNA recognition and to understand the action mode of known methyltransferase inhibitors.
Keywords: RNA processing; RNA virus; biochemistry.
Copyright © 2017 American Society for Microbiology.
Figures




References
-
- Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, Saeed BT, Wahbi A, Saedy A, Al Dabbagh T, Okaili R, Sadat M, Balkhy H. 2015. Severe neurologic syndrome associated with Middle East respiratory syndrome coronavirus (MERS-CoV). Infection 43:495–501. doi: 10.1007/s15010-015-0720-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

