The replication initiator of the cholera pathogen's second chromosome shows structural similarity to plasmid initiators
- PMID: 28031373
- PMCID: PMC5397143
- DOI: 10.1093/nar/gkw1288
The replication initiator of the cholera pathogen's second chromosome shows structural similarity to plasmid initiators
Abstract
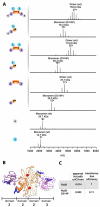
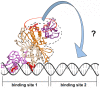
The conserved DnaA-oriC system is used to initiate replication of primary chromosomes throughout the bacterial kingdom; however, bacteria with multipartite genomes evolved distinct systems to initiate replication of secondary chromosomes. In the cholera pathogen, Vibrio cholerae, and in related species, secondary chromosome replication requires the RctB initiator protein. Here, we show that RctB consists of four domains. The structure of its central two domains resembles that of several plasmid replication initiators. RctB contains at least three DNA binding winged-helix-turn-helix motifs, and mutations within any of these severely compromise biological activity. In the structure, RctB adopts a head-to-head dimeric configuration that likely reflects the arrangement in solution. Therefore, major structural reorganization likely accompanies complex formation on the head-to-tail array of binding sites in oriCII. Our findings support the hypothesis that the second Vibrionaceae chromosome arose from an ancestral plasmid, and that RctB may have evolved additional regulatory features.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
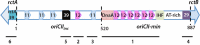




References
-
- Mott M.L., Berger J.M.. DNA replication initiation: mechanisms and regulation in bacteria. Nat. Rev. Microbiol. 2007; 5:343–354. - PubMed
-
- Robinson A., van Oijen A.M.. Bacterial replication, transcription and translation: mechanistic insights from single-molecule biochemical studies. Nat. Rev. Microbiol. 2013; 11:303–315. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

