Neurons derived from different brain regions are inherently different in vitro: a novel multiregional brain-on-a-chip
- PMID: 28031399
- PMCID: PMC5350271
- DOI: 10.1152/jn.00575.2016
Neurons derived from different brain regions are inherently different in vitro: a novel multiregional brain-on-a-chip
Abstract
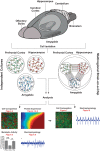
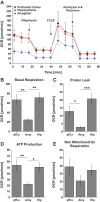
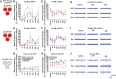
Brain in vitro models are critically important to developing our understanding of basic nervous system cellular physiology, potential neurotoxic effects of chemicals, and specific cellular mechanisms of many disease states. In this study, we sought to address key shortcomings of current brain in vitro models: the scarcity of comparative data for cells originating from distinct brain regions and the lack of multiregional brain in vitro models. We demonstrated that rat neurons from different brain regions exhibit unique profiles regarding their cell composition, protein expression, metabolism, and electrical activity in vitro. In vivo, the brain is unique in its structural and functional organization, and the interactions and communication between different brain areas are essential components of proper brain function. This fact and the observation that neurons from different areas of the brain exhibit unique behaviors in vitro underline the importance of establishing multiregional brain in vitro models. Therefore, we here developed a multiregional brain-on-a-chip and observed a reduction of overall firing activity, as well as altered amounts of astrocytes and specific neuronal cell types compared with separately cultured neurons. Furthermore, this multiregional model was used to study the effects of phencyclidine, a drug known to induce schizophrenia-like symptoms in vivo, on individual brain areas separately while monitoring downstream effects on interconnected regions. Overall, this work provides a comparison of cells from different brain regions in vitro and introduces a multiregional brain-on-a-chip that enables the development of unique disease models incorporating essential in vivo features.NEW & NOTEWORTHY Due to the scarcity of comparative data for cells from different brain regions in vitro, we demonstrated that neurons isolated from distinct brain areas exhibit unique behaviors in vitro. Moreover, in vivo proper brain function is dependent on the connection and communication of several brain regions, underlining the importance of developing multiregional brain in vitro models. We introduced a novel brain-on-a-chip model, implementing essential in vivo features, such as different brain areas and their functional connections.
Keywords: brain-on-a-chip; different brain regions; electrophysiology; metabolism; protein expression.
Copyright © 2017 the American Physiological Society.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

