New Roles of Syntaxin-1A in Insulin Granule Exocytosis and Replenishment
- PMID: 28031464
- PMCID: PMC5313094
- DOI: 10.1074/jbc.M116.769885
New Roles of Syntaxin-1A in Insulin Granule Exocytosis and Replenishment
Abstract
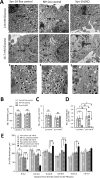
In type-2 diabetes (T2D), severely reduced islet syntaxin-1A (Syn-1A) levels contribute to insulin secretory deficiency. We generated β-cell-specific Syn-1A-KO (Syn-1A-βKO) mice to mimic β-cell Syn-1A deficiency in T2D. Glucose tolerance tests showed that Syn-1A-βKO mice exhibited blood glucose elevation corresponding to reduced blood insulin levels. Perifusion of Syn-1A-βKO islets showed impaired first- and second-phase glucose-stimulated insulin secretion (GSIS) resulting from reduction in readily releasable pool and granule pool refilling. To unequivocally determine the β-cell exocytotic defects caused by Syn-1A deletion, EM and total internal reflection fluorescence microscopy showed that Syn-1A-KO β-cells had a severe reduction in the number of secretory granules (SGs) docked onto the plasma membrane (PM) at rest and reduced SG recruitment to the PM after glucose stimulation, the latter indicating defects in replenishment of releasable pools required to sustain second-phase GSIS. Whereas reduced predocked SG fusion accounted for reduced first-phase GSIS, selective reduction of exocytosis of short-dock (but not no-dock) newcomer SGs accounted for the reduced second-phase GSIS. These Syn-1A actions on newcomer SGs were partly mediated by Syn-1A interactions with newcomer SG VAMP8.
Keywords: SNARE proteins; Syntaxin-1A; Type 2 diabetes; docking; exocytosis; granule replenishment; insulin secretion; newcomer insulin granule; transgenic mice.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






References
-
- Rorsman P., Eliasson L., Renström E., Gromada J., Barg S., and Göpel S. (2000) The cell physiology of biphasic insulin secretion. News Physiol. Sci. 15, 72–77 - PubMed
-
- Gaisano H. Y. (2012) Deploying insulin granule-granule fusion to rescue deficient insulin secretion in diabetes. Diabetologia 55, 877–880 - PubMed
-
- Hong W. (2005) SNAREs and traffic. Biochim. Biophys. Acta 1744, 493–517 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

