Cell-based Fluorescence Complementation Reveals a Role for HIV-1 Nef Protein Dimerization in AP-2 Adaptor Recruitment and CD4 Co-receptor Down-regulation
- PMID: 28031466
- PMCID: PMC5314165
- DOI: 10.1074/jbc.M116.770016
Cell-based Fluorescence Complementation Reveals a Role for HIV-1 Nef Protein Dimerization in AP-2 Adaptor Recruitment and CD4 Co-receptor Down-regulation
Abstract
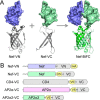
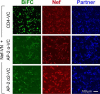
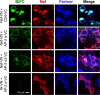
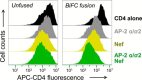
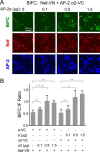
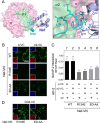
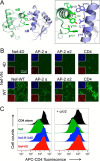
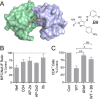
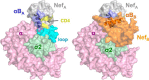
The HIV-1 Nef accessory factor enhances viral infectivity, immune evasion, and AIDS progression. Nef triggers rapid down-regulation of CD4 via the endocytic adaptor protein 2 (AP-2) complex, a process linked to enhanced viral infectivity and immune escape. Here, we describe a bimolecular fluorescence complementation (BiFC) assay to visualize the interaction of Nef with AP-2 and CD4 in living cells. Interacting protein pairs were fused to complementary non-fluorescent fragments of YFP and co-expressed in 293T cells. Nef interactions with both CD4 and AP-2 resulted in complementation of YFP and a bright fluorescent signal by confocal microcopy that localized to the cell periphery. Co-expression of the AP-2 α subunit enhanced the Nef·AP-2 σ2 subunit BiFC signal and vice versa, suggesting that the AP-2 α-σ2 hemicomplex interacts cooperatively with Nef. Mutagenesis of Nef amino acids Arg-134, Glu-174, and Asp-175, which stabilize Nef for AP-2 α-σ2 binding in a recent co-crystal structure, substantially reduced AP-2 interaction without affecting CD4 binding. A dimerization-defective mutant of Nef failed to interact with either CD4 or AP-2 in the BiFC assay, indicating that Nef quaternary structure is required for CD4 and AP-2 recruitment as well as CD4 down-regulation. A small molecule previously shown to bind the Nef dimerization interface also reduced Nef interactions with AP-2 and CD4 and restored CD4 expression to the surface of HIV-infected cells. Our findings provide a mechanistic explanation for previous observations that dimerization-defective Nef mutants fail to down-regulate CD4 and validate the Nef dimerization interface as a target site for antiretroviral drug development.
Keywords: AP-2; Nef; bimolecular fluorescence complementation; cluster of differentiation 4 (CD4); dimerization; endocytosis; human immunodeficiency virus (HIV); protein-protein interaction.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
-
- Kestler H. W. 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., and Desrosiers R. C. (1991) Importance of the nef gene for maintenance of high viral loads and for development of AIDS. Cell 65, 651–662 - PubMed
-
- Deacon N. J., Tsykin A., Solomon A., Smith K., Ludford-Menting M., Hooker D. J., McPhee D. A., Greenway A. L., Ellett A., Chatfield C., Lawson V. A., Crowe S., Maerz A., Sonza S., Learmont J., et al. (1995) Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270, 988–991 - PubMed
-
- Kirchhoff F., Greenough T. C., Brettler D. B., Sullivan J. L., and Desrosiers R. C. (1995) Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332, 228–232 - PubMed
-
- Jolicoeur P. (2011) The CD4C/HIV(Nef)transgenic model of AIDS. Curr. HIV Res. 9, 524–530 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

