Lipin-2 regulates NLRP3 inflammasome by affecting P2X7 receptor activation
- PMID: 28031477
- PMCID: PMC5294860
- DOI: 10.1084/jem.20161452
Lipin-2 regulates NLRP3 inflammasome by affecting P2X7 receptor activation
Abstract
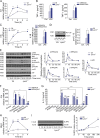
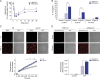
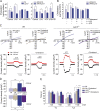
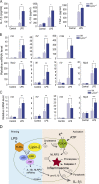
Mutations in human LPIN2 produce a disease known as Majeed syndrome, the clinical manifestations of which are ameliorated by strategies that block IL-1β or its receptor. However the role of lipin-2 during IL-1β production remains elusive. We show here that lipin-2 controls excessive IL-1β formation in primary human and mouse macrophages by several mechanisms, including activation of the inflammasome NLRP3. Lipin-2 regulates MAPK activation, which mediates synthesis of pro-IL-1β during inflammasome priming. Lipin-2 also inhibits the activation and sensitization of the purinergic receptor P2X7 and K+ efflux, apoptosis-associated speck-like protein with a CARD domain oligomerization, and caspase-1 processing, key events during inflammasome activation. Reduced levels of lipin-2 in macrophages lead to a decrease in cellular cholesterol levels. In fact, restoration of cholesterol concentrations in cells lacking lipin-2 decreases ion currents through the P2X7 receptor, and downstream events that drive IL-1β production. Furthermore, lipin-2-deficient mice exhibit increased sensitivity to high lipopolysaccharide doses. Collectively, our results unveil lipin-2 as a critical player in the negative regulation of NLRP3 inflammasome.
© 2017 Lordén et al.
Figures





References
-
- Baldassare, J.J., Bi Y., and Bellone C.J.. 1999. The role of p38 mitogen-activated protein kinase in IL-1 β transcription. J. Immunol. 162:5367–5373. - PubMed
-
- Basak, C., Pathak S.K., Bhattacharyya A., Mandal D., Pathak S., and Kundu M.. 2005. NF-κB- and C/EBPβ-driven interleukin-1β gene expression and PAK1-mediated caspase-1 activation play essential roles in interleukin-1β release from Helicobacter pylori lipopolysaccharide-stimulated macrophages. J. Biol. Chem. 280:4279–4288. 10.1074/jbc.M412820200 - DOI - PubMed
-
- Bauernfeind, F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B.G., Fitzgerald K.A., et al. . 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183:787–791. 10.4049/jimmunol.0901363 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

