Cellular stress responses in protein misfolding diseases
- PMID: 28031871
- PMCID: PMC5137871
- DOI: 10.4155/fso.15.42
Cellular stress responses in protein misfolding diseases
Abstract
Many human diseases, particularly neurodegenerative diseases, are associated with protein misfolding. Cellular protein quality control includes all processes that ensure proper protein folding and thus prevent the toxic consequences of protein misfolding. The heat shock response (HSR) and the unfolded protein response (UPR) are major stress response pathways within protein quality control that antagonize protein misfolding in the cytosol and the endoplasmic reticulum, respectively. Huntington's disease is an inherited neurodegenerative disease caused by the misfolding of an abnormally expanded polyglutamine (polyQ) region in the protein huntingtin (Htt), polyQHtt. Using Huntington's disease as a paradigm, I review here the central role of both the HSR and the UPR in defining the toxicity associated with polyQHtt in Huntington's disease. These findings may begin to unravel a previously unappreciated cooperation between different stress response pathways in cells expressing misfolded proteins and consequently in neurodegenerative diseases.
Keywords: cellular protein quality control; heat shock response; polyQ expansion diseases; protein misfolding; stress response pathways; unfolded protein response.
Conflict of interest statement
Financial & competing interests disclosure The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Figures
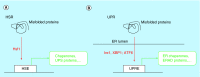
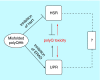
References
-
- Soto C, Estrada LD. Protein misfolding and neurodegeneration. Arch. Neurol. 2008;65:184–189. - PubMed
-
- Gusella JF, MacDonald ME. Huntington's disease: seeing the pathogenic process through a genetic lens. Trends Biochem. Sci. 2006;31:533–540. - PubMed
-
- Cattaneo E, Zuccato C, Tartari M. Normal huntingtin function: an alternative approach to Huntington's disease. Nat. Rev. Neurosci. 2005;6:919–930. - PubMed
-
- Walker FO. Huntington's disease. Lancet. 2007;369:218–228. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
