Possible roles of amyloids in malaria pathophysiology
- PMID: 28031872
- PMCID: PMC5137859
- DOI: 10.4155/fso.15.43
Possible roles of amyloids in malaria pathophysiology
Abstract
The main therapeutic and prophylactic tools against malaria have been locked for more than a century in the classical approaches of using drugs targeting metabolic processes of the causing agent, the protist Plasmodium spp., and of designing vaccines against chosen antigens found on the parasite's surface. Given the extraordinary resources exhibited by Plasmodium to escape these traditional strategies, which have not been able to free humankind from the scourge of malaria despite much effort invested in them, new concepts have to be explored in order to advance toward eradication of the disease. In this context, amyloid-forming proteins and peptides found in the proteome of the pathogen should perhaps cease being regarded as mere anomalous molecules. Their likely functionality in the pathophysiology of Plasmodium calls for attention being paid to them as a possible Achilles' heel of malaria. Here we will give an overview of Plasmodium-encoded amyloid-forming polypeptides as potential therapeutic targets and toxic elements, particularly in relation to cerebral malaria and the blood-brain barrier function. We will also discuss the recent finding that the genome of the parasite contains an astonishingly high proportion of prionogenic domains.
Keywords: amyloids; intrinsically unstructured proteins; malaria; prions.
Conflict of interest statement
Financial & competing interests disclosure This work was supported by grants BIO2011–25039, BIO2014–52872-R and BFU2012–33932, from the Ministerio de Economía y Competitividad, Spain, which included FEDER funds, and 2014-SGR-938 from the Generalitat de Catalunya, Spain. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures
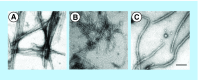
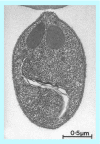

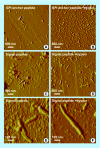

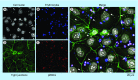
References
-
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124(4):755–766. - PubMed
-
- Feng ZP, Zhang X, Han P, Arora N, Anders RF, Norton RS. Abundance of intrinsically unstructured proteins in P. falciparum and other apicomplexan parasite proteomes. Mol. Biochem. Parasitol. 2006;150(2):256–267. - PubMed
-
- Iriemenam NC, Khirelsied AH, Nasr A, et al. Antibody responses to a panel of Plasmodium falciparum malaria blood-stage antigens in relation to clinical disease outcome in Sudan. Vaccine. 2009;27(1):62–71. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
