Efficacy and toxicity profile of maintenance pemetrexed in patients with stage IV adenocarcinoma lung in Indian population
- PMID: 28032090
- PMCID: PMC5184760
- DOI: 10.4103/2278-330X.195345
Efficacy and toxicity profile of maintenance pemetrexed in patients with stage IV adenocarcinoma lung in Indian population
Abstract
Context: Lung cancer has been the most common cancer in the world for several decades. Pemetrexed is recommended as an option for the maintenance treatment in metastatic adenocarcinoma lung, if disease has not progressed immediately following platinum-based chemotherapy.
Aims: To study efficacy and toxicity profile of pemetrexed as a maintenance chemotherapeutic agent in patients with stage IV adenocarcinoma lung, not progressing after first line chemotherapy. Settings and Design: This was an observational, prospective. We enrolled patients with stage IV adenocarcinoma lung who has not progressed on first line chemotherapy, from September 2013 to August 2014 at a tertiary care cancer institute in North India.
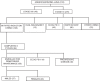
Materials and methods: In all, 108 patients with stage IV adenocarcinoma lung were started on induction pemetrexed/platinum chemotherapy. 60 patients with no disease progression & ECOG PS 0-2 were started on Pemetrexed maintenance. Progression free survival (PFS) and toxicity profile were recorded.
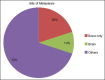
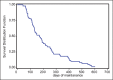
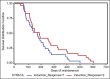
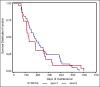
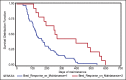
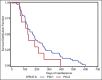
Results: The mean number of maintenance cycles was 8.3 (range 2-28). 13 (21.6%) patients took >10 maintenance cycles. Pemetrexed maintenance therapy resulted in progression free survival (PFS) of 5.4 months. PFS on pemetrexed was consistent for all patient subgroups, including induction response: complete/partial responders (n-31) and stable disease (n-29). 14 patients had grade III/IV adverse events with anemia being the most common in 3/60 patients (5%). 3 patients (5%) developed renal dysfunction out of which 1 was grade III.
Conclusions: Pemetrexed continuation maintenance chemotherapy is active and well tolerated. Pemetrexed maintenance should be considered in patients with advanced adenocarcinoma lung patients who have not progressed on completion of induction chemotherapy.
Keywords: Adenocacinoma lung; pemetrexed; pemetrexed maintenance.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. - PubMed
-
- Tanei T, Shimomura A, Shimazu K, Nakayama T, Kim SJ, Iwamoto T, et al. Prognostic significance of Ki67 index after neoadjuvant chemotherapy in breast cancer. Eur J Surg Oncol. 2011;37:155–61. - PubMed
-
- Delbaldo C, Michiels S, Rolland E, Syz N, Soria JC, Le Chevalier T, et al. Second or third additional chemotherapy drug for non-small cell lung cancer in patients with advanced disease. Cochrane Database Syst Rev. 2007;4:CD004569. - PubMed
-
- Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. - PubMed
-
- Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

