Regulation of ER stress-induced autophagy by GSK3β-TIP60-ULK1 pathway
- PMID: 28032867
- PMCID: PMC5260977
- DOI: 10.1038/cddis.2016.423
Regulation of ER stress-induced autophagy by GSK3β-TIP60-ULK1 pathway
Abstract
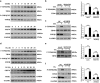
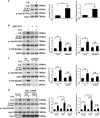
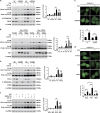
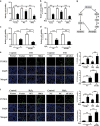
Endoplasmic reticulum (ER) stress is involved in many cellular processes. Emerging evidence suggests that ER stress can trigger autophagy; however, the mechanisms by which ER stress regulates autophagy and its role in this condition are not fully understood. HIV Tat-interactive protein, 60 kDa (TIP60) is a newly discovered acetyltransferase that can modulate autophagy flux by activating ULK1 upon growth factor deprivation. In this study, we investigated the mechanisms by which ER stress induces autophagy. We showed that ER stress activates glycogen synthase kinase-3β (GSK3β). This led to a GSK3β-dependent phosphorylation of TIP60, triggering a TIP60-mediated acetylation of ULK1 and activation of autophagy. Inhibition of either GSK3β or TIP60 acetylation activities significantly attenuated ER stress-induced autophagy. Moreover, enhancing the level of TIP60 attenuated the level of CHOP after ER stress, and reduced the ER stress-induced cell death. In contrast, expression of TIP60 mutant that could not be phosphorylated by GSK3β exacerbated the generation of CHOP and increased the ER stress-induced cell death. These findings reveal that ER stress engages the GSK3β-TIP60-ULK1 pathway to increase autophagy. Attenuation of this pathway renders cells more sensitive to and increases the toxicity of ER stress.
Figures



Similar articles
-
GSK3-TIP60-ULK1 signaling pathway links growth factor deprivation to autophagy.Science. 2012 Apr 27;336(6080):477-81. doi: 10.1126/science.1217032. Science. 2012. PMID: 22539723
-
The unc-51 like autophagy activating kinase 1-autophagy related 13 complex has distinct functions in tunicamycin-treated cells.Biochem Biophys Res Commun. 2020 Apr 9;524(3):744-749. doi: 10.1016/j.bbrc.2020.01.160. Epub 2020 Feb 5. Biochem Biophys Res Commun. 2020. PMID: 32035621
-
Inhibition of autophagy enhances adenosine‑induced apoptosis in human hepatoblastoma HepG2 cells.Oncol Rep. 2019 Feb;41(2):829-838. doi: 10.3892/or.2018.6899. Epub 2018 Nov 30. Oncol Rep. 2019. PMID: 30535464 Free PMC article.
-
The unfolded protein response as a target for anticancer therapeutics.Crit Rev Oncol Hematol. 2018 Jul;127:66-79. doi: 10.1016/j.critrevonc.2018.05.003. Epub 2018 May 26. Crit Rev Oncol Hematol. 2018. PMID: 29891114 Review.
-
Post-translational modifications of epigenetic modifier TIP60: their role in cellular functions and cancer.Epigenetics Chromatin. 2025 Apr 4;18(1):18. doi: 10.1186/s13072-025-00572-y. Epigenetics Chromatin. 2025. PMID: 40186325 Free PMC article. Review.
Cited by
-
Phosphorylation of TIP60 Suppresses 53BP1 Localization at DNA Damage Sites.Mol Cell Biol. 2018 Dec 11;39(1):e00209-18. doi: 10.1128/MCB.00209-18. Print 2019 Jan 1. Mol Cell Biol. 2018. PMID: 30297459 Free PMC article.
-
The crosstalk of NAD, ROS and autophagy in cellular health and ageing.Biogerontology. 2020 Jun;21(3):381-397. doi: 10.1007/s10522-020-09864-0. Epub 2020 Mar 3. Biogerontology. 2020. PMID: 32124104 Free PMC article. Review.
-
Claudin-1 silencing increases sensitivity of liver cancer HepG2 cells to 5-fluorouracil by inhibiting autophagy.Oncol Lett. 2019 Dec;18(6):5709-5716. doi: 10.3892/ol.2019.10967. Epub 2019 Oct 8. Oncol Lett. 2019. PMID: 31788043 Free PMC article.
-
Loss of Drosha underlies dopaminergic neuron toxicity in models of Parkinson's disease.Cell Death Dis. 2018 Jun 7;9(6):693. doi: 10.1038/s41419-018-0716-5. Cell Death Dis. 2018. PMID: 29880811 Free PMC article.
-
Selective autophagy of intracellular organelles: recent research advances.Theranostics. 2021 Jan 1;11(1):222-256. doi: 10.7150/thno.49860. eCollection 2021. Theranostics. 2021. PMID: 33391472 Free PMC article.
References
-
- Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 2006; 441: 513–517. - PubMed
-
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306: 457–461. - PubMed
-
- Hoozemans JJ, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P et al. The unfolded protein response is activated in Alzheimer's disease. Acta Neuropathol 2005; 110: 165–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

