Production of Polyclonal Antibody to the HPV58 E7 Protein and Its Detection in Cervical Cancer
- PMID: 28033368
- PMCID: PMC5199089
- DOI: 10.1371/journal.pone.0169138
Production of Polyclonal Antibody to the HPV58 E7 Protein and Its Detection in Cervical Cancer
Abstract
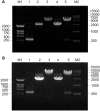
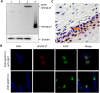
The persistent infection of high-risk human papillomavirus (HPV) is one of the most common causes of cervical cancer worldwide, and HPV type 58 is the third most common HPV type in eastern Asia. The E7 oncoprotein is constitutively expressed in HPV58-associated cervical cancer cells and plays a key role during tumorigenesis. To study the biological function of HPV58 E7 and to characterize E7 protein-host cell interactions, we cloned the human HPV58 E7 gene and produced specific E7 antibodies. The HPV58 E7 gene was cloned into a prokaryotic expression vector, pGEX-4T2. The recombinant plasmid pGEX-4T2-(HPV58-E7) was transformed into Escherichia coli DH5α and expressed as a fusion protein containing a GST tag. After purification and removal of the GST affinity tag, the E7 protein was used as an antigen for the production of antiserum in rabbits. The specificity of the purified HPV58 E7 antibody was detected by western blotting, immunofluorescence and immunohistochemistry analysis. These methods demonstrated that the polyclonal antibody could specifically recognize the endogenous and the recombinant HPV58 E7 proteins. Immunohistochemistry analysis indicated that the E7 protein was localized in the nucleus of cervical cancer cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
HPV58 E7 Protein Expression Profile in Cervical Cancer and CIN with Immunohistochemistry.J Cancer. 2021 Jan 18;12(6):1722-1728. doi: 10.7150/jca.50816. eCollection 2021. J Cancer. 2021. PMID: 33613760 Free PMC article.
-
[Expression of HPV16 E7 protein and preparation of its polyclonal antibody].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014 Feb;30(2):167-70. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014. PMID: 24491058 Chinese.
-
[Detection of HPV 58 and cloning of its E7 gene in cervical cancer].Zhonghua Zhong Liu Za Zhi. 2004 Sep;26(9):543-6. Zhonghua Zhong Liu Za Zhi. 2004. PMID: 15555285 Chinese.
-
Human Papillomavirus E7 Oncoprotein Promotes Proliferation and Migration through the Transcription Factor E2F1 in Cervical Cancer Cells.Anticancer Agents Med Chem. 2021;21(13):1689-1696. doi: 10.2174/1871520620666201106085227. Anticancer Agents Med Chem. 2021. PMID: 33155930
-
[Human papillomavirus E7 oncoprotein and its role in the cell transformation].Rev Med Inst Mex Seguro Soc. 2015;53 Suppl 2:S172-7. Rev Med Inst Mex Seguro Soc. 2015. PMID: 26462513 Review. Spanish.
Cited by
-
Production of antibody against elephant endotheliotropic herpesvirus (EEHV) unveils tissue tropisms and routes of viral transmission in EEHV-infected Asian elephants.Sci Rep. 2018 Mar 16;8(1):4675. doi: 10.1038/s41598-018-22968-5. Sci Rep. 2018. PMID: 29549315 Free PMC article.
-
Immunodiagnosis and Immunotherapeutics Based on Human Papillomavirus for HPV-Induced Cancers.Front Immunol. 2021 Jan 8;11:586796. doi: 10.3389/fimmu.2020.586796. eCollection 2020. Front Immunol. 2021. PMID: 33488587 Free PMC article. Review.
-
Anatomical variations, treatment and outcomes of Herlyn-Werner-Wunderlich syndrome: a literature review of 1673 cases.Arch Gynecol Obstet. 2023 Nov;308(5):1409-1417. doi: 10.1007/s00404-022-06856-y. Epub 2023 Feb 24. Arch Gynecol Obstet. 2023. PMID: 36823415
-
Digital RNA Sequencing of Human Epidermal Keratinocytes Carrying Human Papillomavirus Type 16 E7.Front Genet. 2020 Aug 5;11:819. doi: 10.3389/fgene.2020.00819. eCollection 2020. Front Genet. 2020. PMID: 32849815 Free PMC article.
-
HPV58 E7 Protein Expression Profile in Cervical Cancer and CIN with Immunohistochemistry.J Cancer. 2021 Jan 18;12(6):1722-1728. doi: 10.7150/jca.50816. eCollection 2021. J Cancer. 2021. PMID: 33613760 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

