Wide spectrum of NR5A1-related phenotypes in 46,XY and 46,XX individuals
- PMID: 28033660
- PMCID: PMC5347970
- DOI: 10.1002/bdrc.21145
Wide spectrum of NR5A1-related phenotypes in 46,XY and 46,XX individuals
Abstract
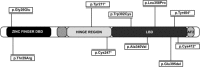
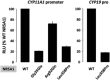
Steroidogenic factor 1 (NR5A1, SF-1, Ad4BP) is a transcriptional regulator of genes involved in adrenal and gonadal development and function. Mutations in NR5A1 have been among the most frequently identified genetic causes of gonadal development disorders and are associated with a wide phenotypic spectrum. In 46,XY individuals, NR5A1-related phenotypes may range from disorders of sex development (DSD) to oligo/azoospermia, and in 46,XX individuals, from 46,XX ovotesticular and testicular DSD to primary ovarian insufficiency (POI). The most common 46,XY phenotype is atypical or female external genitalia with clitoromegaly, palpable gonads, and absence of Müllerian derivatives. Notably, an undervirilized external genitalia is frequently seen at birth, while spontaneous virilization may occur later, at puberty. In 46,XX individuals, NR5A1 mutations are a rare genetic cause of POI, manifesting as primary or secondary amenorrhea, infertility, hypoestrogenism, and elevated gonadotropin levels. Mothers and sisters of 46,XY DSD patients carrying heterozygous NR5A1 mutations may develop POI, and therefore require appropriate counseling. Moreover, the recurrent heterozygous p.Arg92Trp NR5A1 mutation is associated with variable degrees of testis development in 46,XX patients. A clear genotype-phenotype correlation is not seen in patients bearing NR5A1 mutations, suggesting that genetic modifiers, such as pathogenic variants in other testis/ovarian-determining genes, may contribute to the phenotypic expression. Here, we review the published literature on NR5A1-related disease, and discuss our findings at a single tertiary center in Brazil, including ten novel NR5A1 mutations identified in 46,XY DSD patients. The ever-expanding phenotypic range associated with NR5A1 variants in XY and XX individuals confirms its pivotal role in reproductive biology, and should alert clinicians to the possibility of NR5A1 defects in a variety of phenotypes presenting with gonadal dysfunction. Birth Defects Research (Part C) 108:309-320, 2016. © 2016 The Authors Birth Defects Research Part C: Embryo Today: Reviews Published by Wiley Periodicals, Inc.
Keywords: NR5A1 gene; adrenal insufficiency; disorders of sex development; gonadal dysgenesis; ovotestis; primary ovarian failure.
© 2016 The Authors Birth Defects Research Part C: Embryo Today: Reviews Published by Wiley Periodicals, Inc.
Figures


References
-
- Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. 1999. A mutation in the gene encoding steroidogenic factor‐1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 22(2):125–126. - PubMed
-
- Achermann JC, Ozisik G, Ito M, et al. 2002. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor‐1, in a dose‐dependent manner. J Clin Endocrinol Metab 87(4):1829–1833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

