Determinants of denervation-independent depletion of putamen dopamine in Parkinson's disease and multiple system atrophy
- PMID: 28034624
- PMCID: PMC6319359
- DOI: 10.1016/j.parkreldis.2016.12.011
Determinants of denervation-independent depletion of putamen dopamine in Parkinson's disease and multiple system atrophy
Erratum in
-
Corrigendum to "Determinants of denervation-independent depletion of putamen dopamine in Parkinson's disease and multiple system atrophy" [Parkinsonism Relat. Disord. 35 (2017) 88-91].Parkinsonism Relat Disord. 2017 Jun;39:95. doi: 10.1016/j.parkreldis.2017.03.018. Epub 2017 Apr 6. Parkinsonism Relat Disord. 2017. PMID: 28392299 No abstract available.
Abstract
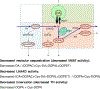
Background: Severe putamen dopamine depletion characterizes Parkinson's disease (PD) and multiple system atrophy (MSA). The extent of the depletion is greater than can be accounted for by loss of nigrostriatal dopaminergic terminals alone. We used putamen tissue levels and ratios of cysteinyl and parent catechols to explore possible denervation-independent abnormalities of dopamine synthesis and fate in PD and MSA. 5-S-Cysteinyldopa (Cys-DOPA) is produced from spontaneous oxidation of DOPA and 5-S-cysteinyldopamine (Cys-DA) from spontaneous oxidation of DA.
Methods: Post-mortem putamen tissue samples from 17 PD and 25 MSA patients and 30 controls were assayed for endogenous catechols including DA, its cytoplasmic metabolites (Cys-DA, 3,4-dihydroxyphenylacetic acid, 3,4-dihydroxyphenylethanol, and 3,4-dihydroxyphenylacetaldehyde), and tyrosine hydroxylation products proximal to DA (DOPA and Cys-DOPA).
Results: The PD and MSA groups did not differ in mean values of parent or cysteinyl catechols, and the data for the two groups were lumped. In the patients an index of vesicular storage of DA (the ratio of DA to the sum of its cytoplasmic metabolites) averaged 54% of control (p = 0.001), and an index of L-aromatic-amino-acid decarboxylase (LAAAD) activity (the ratio of DA and the sum of its cytoplasmic metabolites to the sum of DOPA + Cys-DOPA) averaged 21% of control (p < 0.0001). An index of innervation (the sum of DOPA + Cys-DOPA) averaged 63% of control (p = 0.01).
Interpretation: Based on patterns of parent and cysteinyl catechols in putamen, PD and MSA involve decreased vesicular uptake and decreased LAAAD activity in the residual dopaminergic terminals. The combination seems to contribute importantly to dopamine depletion in these diseases.
Keywords: Cysteinyl-DOPA; Cysteinyl-dopamine; Multiple system atrophy; Parkinson’s disease.
Copyright © 2016. Published by Elsevier Ltd.
Conflict of interest statement
Conflicts of interest
The Authors have no conflicts of interest to disclose.
Figures
References
-
- Kish SJ, Shannak K, Hornykiewicz O Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications, N. Engl. J. Med, 318 (1988), pp. 876–880 - PubMed
-
- DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ, Ahlskog JE, Dickson DW Incidental Lewy body disease and preclinical Parkinson disease, Arch. Neurol, 65 (2008), pp. 1074–1080 - PubMed
-
- Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, Blessing WW, Geffen LB Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease Ann. Neurol, 27 (1990), pp. 373–385 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

