Matrix stiffening promotes a tumor vasculature phenotype
- PMID: 28034921
- PMCID: PMC5255592
- DOI: 10.1073/pnas.1613855114
Matrix stiffening promotes a tumor vasculature phenotype
Abstract
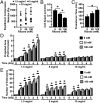
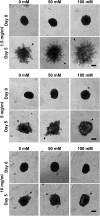
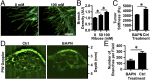
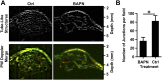
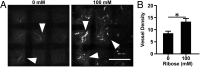
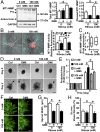
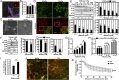
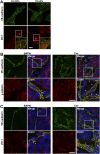
Tumor microvasculature tends to be malformed, more permeable, and more tortuous than vessels in healthy tissue, effects that have been largely attributed to up-regulated VEGF expression. However, tumor tissue tends to stiffen during solid tumor progression, and tissue stiffness is known to alter cell behaviors including proliferation, migration, and cell-cell adhesion, which are all requisite for angiogenesis. Using in vitro, in vivo, and ex ovo models, we investigated the effects of matrix stiffness on vessel growth and integrity during angiogenesis. Our data indicate that angiogenic outgrowth, invasion, and neovessel branching increase with matrix cross-linking. These effects are caused by increased matrix stiffness independent of matrix density, because increased matrix density results in decreased angiogenesis. Notably, matrix stiffness up-regulates matrix metalloproteinase (MMP) activity, and inhibiting MMPs significantly reduces angiogenic outgrowth in stiffer cross-linked gels. To investigate the functional significance of altered endothelial cell behavior in response to matrix stiffness, we measured endothelial cell barrier function on substrates mimicking the stiffness of healthy and tumor tissue. Our data indicate that barrier function is impaired and the localization of vascular endothelial cadherin is altered as function of matrix stiffness. These results demonstrate that matrix stiffness, separately from matrix density, can alter vascular growth and integrity, mimicking the changes that exist in tumor vasculature. These data suggest that therapeutically targeting tumor stiffness or the endothelial cell response to tumor stiffening may help restore vessel structure, minimize metastasis, and aid in drug delivery.
Keywords: endothelial cells; extracellular matrix; glycation; tumor stiffness; vascular permeability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Matrix Biology Highlights.Matrix Biol. 2017 May;59:1-2. doi: 10.1016/j.matbio.2017.03.001. Matrix Biol. 2017. PMID: 28335831 No abstract available.
References
-
- Trédan O, Galmarini CM, Patel K, Tannock IF, Tredan O. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. - PubMed
-
- Carmeliet P. VEGF gene therapy: Stimulating angiogenesis or angioma-genesis? Nat Med. 2000;6(10):1102–1103. - PubMed
-
- Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

