Structural Insights into c-Myc-interacting Zinc Finger Protein-1 (Miz-1) Delineate Domains Required for DNA Scanning and Sequence-specific Binding
- PMID: 28035002
- PMCID: PMC5336166
- DOI: 10.1074/jbc.M116.748699
Structural Insights into c-Myc-interacting Zinc Finger Protein-1 (Miz-1) Delineate Domains Required for DNA Scanning and Sequence-specific Binding
Abstract
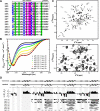
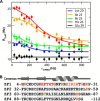
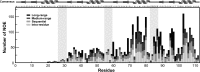
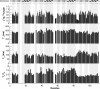
c-Myc-interacting zinc finger protein-1 (Miz-1) is a poly-Cys2His2 zinc finger (ZF) transcriptional regulator of many cell cycle genes. A Miz-1 DNA sequence consensus has recently been identified and has also unveiled Miz-1 functions in other cellular processes, underscoring its importance in the cell. Miz-1 contains 13 ZFs, but it is unknown why Miz-1 has so many ZFs and whether they recognize and bind DNA sequences in a typical fashion. Here, we used NMR to deduce the role of Miz-1 ZFs 1-4 in detecting the Miz-1 consensus sequence and preventing nonspecific DNA binding. In the construct containing the first 4 ZFs, we observed that ZFs 3 and 4 form an unusual compact and stable structure that restricts their motions. Disruption of this compact structure by an electrostatically mismatched A86K mutation profoundly affected the DNA binding properties of the WT construct. On the one hand, Miz1-4WT was found to bind the Miz-1 DNA consensus sequence weakly and through ZFs 1-3 only. On the other hand, the four ZFs in the structurally destabilized Miz1-4A86K mutant bound to the DNA consensus with a 30-fold increase in affinity (100 nm). The formation of such a thermodynamically stable but nonspecific complex is expected to slow down the rate of DNA scanning by Miz-1 during the search for its consensus sequence. Interestingly, we found that the motif stabilizing the compact structure between ZFs 3 and 4 is conserved and enriched in other long poly-ZF proteins. As discussed in detail, our findings support a general role of compact inter-ZF structures in minimizing the formation of off-target DNA complexes.
Keywords: fluorescence anisotropy; nuclear magnetic resonance (NMR); protein dynamic; protein-DNA interaction; protein-protein interaction; structural biology; transcription factor; zinc finger.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Seoane J., Le H. V., and Massagué J. (2002) Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature 419, 729–734 - PubMed
-
- Staller P., Peukert K., Kiermaier A., Seoane J., Lukas J., Karsunky H., Möröy T., Bartek J., Massague J., Hänel F., and Eilers M. (2001) Repression of p15INK4b expression by Myc through association with Miz-1. Nat. Cell Biol. 3, 392–399 - PubMed
-
- van Riggelen J., Müller J., Otto T., Beuger V., Yetil A., Choi P. S., Kosan C., Möröy T., Felsher D. W., and Eilers M. (2010) The interaction between Myc and Miz1 is required to antagonize TGFβ-dependent autocrine signaling during lymphoma formation and maintenance. Genes Dev. 24, 1281–1294 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

