Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2
- PMID: 28035139
- PMCID: PMC5339831
- DOI: 10.1038/cr.2016.159
Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2
Abstract
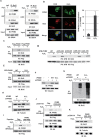
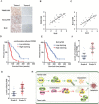
Pyruvate kinase M2 isoform (PKM2) catalyzes the last step of glycolysis and plays an important role in tumor cell proliferation. Recent studies have reported that PKM2 also regulates apoptosis. However, the mechanisms underlying such a role of PKM2 remain elusive. Here we show that PKM2 translocates to mitochondria under oxidative stress. In the mitochondria, PKM2 interacts with and phosphorylates Bcl2 at threonine (T) 69. This phosphorylation prevents the binding of Cul3-based E3 ligase to Bcl2 and subsequent degradation of Bcl2. A chaperone protein, HSP90α1, is required for this function of PKM2. HSP90α1's ATPase activity launches a conformational change of PKM2 and facilitates interaction between PKM2 and Bcl2. Replacement of wild-type Bcl2 with phosphorylation-deficient Bcl2 T69A mutant sensitizes glioma cells to oxidative stress-induced apoptosis and impairs brain tumor formation in an orthotopic xenograft model. Notably, a peptide that is composed of the amino acid residues from 389 to 405 of PKM2, through which PKM2 binds to Bcl2, disrupts PKM2-Bcl2 interaction, promotes Bcl2 degradation and impairs brain tumor growth. In addition, levels of Bcl2 T69 phosphorylation, conformation-altered PKM2 and Bcl2 protein correlate with one another in specimens of human glioblastoma patients. Moreover, levels of Bcl2 T69 phosphorylation and conformation-altered PKM2 correlate with both grades and prognosis of glioma malignancy. Our findings uncover a novel mechanism through which mitochondrial PKM2 phosphorylates Bcl2 and inhibits apoptosis directly, highlight the essential role of PKM2 in ROS adaptation of cancer cells, and implicate HSP90-PKM2-Bcl2 axis as a potential target for therapeutic intervention in glioblastoma.
Figures





References
-
- Visconti R, Grieco D. New insights on oxidative stress in cancer. Curr Opin Drug Discov Devel 2009; 12:240–245. - PubMed
-
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009; 8:579–591. - PubMed
-
- Schneider BL, Kulesz-Martin M. Destructive cycles: the role of genomic instability and adaptation in carcinogenesis. Carcinogenesis 2004; 25:2033–2044. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

