High-resolution electrical mapping of porcine gastric slow-wave propagation from the mucosal surface
- PMID: 28035728
- PMCID: PMC5393964
- DOI: 10.1111/nmo.13010
High-resolution electrical mapping of porcine gastric slow-wave propagation from the mucosal surface
Abstract
Background: Gastric motility is coordinated by bioelectrical slow waves, and gastric dysrhythmias are reported in motility disorders. High-resolution (HR) mapping has advanced the accurate assessment of gastric dysrhythmias, offering promise as a diagnostic technique. However, HR mapping has been restricted to invasive surgical serosal access. This study investigates the feasibility of HR mapping from the gastric mucosal surface.
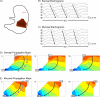
Methods: Experiments were conducted in vivo in 14 weaner pigs. Reference serosal recordings were performed with flexible-printed-circuit (FPC) arrays (128-192 electrodes). Mucosal recordings were performed by two methods: (i) FPC array aligned directly opposite the serosal array, and (ii) cardiac mapping catheter modified for gastric mucosal recordings. Slow-wave propagation and morphology characteristics were quantified and compared between simultaneous serosal and mucosal recordings.
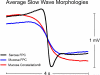
Key results: Slow-wave activity was consistently recorded from the mucosal surface from both electrode arrays. Mucosally recorded slow-wave propagation was consistent with reference serosal activation pattern, frequency (P≥.3), and velocity (P≥.4). However, mucosally recorded slow-wave morphology exhibited reduced amplitude (65-72% reduced, P<.001) and wider downstroke width (18-31% wider, P≤.02), compared to serosal data. Dysrhythmias were successfully mapped and classified from the mucosal surface, accorded with serosal data, and were consistent with known dysrhythmic mechanisms in the porcine model.
Conclusions & inferences: High-resolution gastric electrical mapping was achieved from the mucosal surface, and demonstrated consistent propagation characteristics with serosal data. However, mucosal signal morphology was attenuated, demonstrating necessity for optimized electrode designs and analytical algorithms. This study demonstrates feasibility of endoscopic HR mapping, providing a foundation for advancement of minimally invasive spatiotemporal gastric mapping as a clinical and scientific tool.
Keywords: dysmotility; electrophysiology; endoscopy; motility; stomach.
© 2016 John Wiley & Sons Ltd.
Figures




References
-
- Huizinga JD, Lammers WJEP. ut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1–8. - PubMed
-
- Lin X, Chen JZ. Abnormal gastric slow waves in patients with functional dyspepsia assessed by multichannel electrogastrography. Am J Physiol Gastrointest Liver Physiol. 2001;280(6):G1370–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

