Investigations into Retinal Pathology in the Early Stages of a Mouse Model of Alzheimer's Disease
- PMID: 28035930
- PMCID: PMC5271427
- DOI: 10.3233/JAD-160823
Investigations into Retinal Pathology in the Early Stages of a Mouse Model of Alzheimer's Disease
Abstract
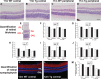
There is increasing recognition that visual performance is impaired in early stages of Alzheimer's disease (AD); however, no consensus exists as to the mechanisms underlying this visual dysfunction, in particular regarding the timing, nature, and extent of retinal versus cortical pathology. If retinal pathology presents sufficiently early, it offers great potential as a source of novel biomarkers for disease diagnosis. The current project utilized an array of immunochemical and molecular tools to perform a characterization of retinal pathology in the early stages of disease progression using a well-validated mouse model of AD (APPSWE/PS1ΔE9). Analytical endpoints included examination of aberrant amyloid and tau in the retina, quantification of any neuronal degeneration, delineation of cellular stress responses of neurons and particularly glial cells, and investigation of oxidative stress. Brain, eyes, and optic nerves were taken from transgenic and wild-type mice of 3 to 12 months of age and processed for immunohistochemistry, qPCR, or western immunoblotting. The results revealed robust expression of the human APP transgene in the retinas of transgenic mice, but a lack of identifiable retinal pathology during the period when amyloid deposits were dramatically escalating in the brain. We were unable to demonstrate the presence of amyloid plaques, dystrophic neurites, neuronal loss, macro- or micro-gliosis, aberrant cell cycle re-entry, oxidative stress, tau hyperphosphorylation, or upregulations of proinflammatory cytokines or stress signaling molecules in the retina. The overall results do not support the hypothesis that detectable retinal pathology occurs concurrently with escalating amyloid deposition in the brains of APPSWE/PS1ΔE9 mice.
Keywords: Alzheimer’s disease; early biomarker; microglia; müller cell; retina; retinal ganglion cell.
Figures










References
-
- Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Science 314, 777–781. - PubMed
-
- Krantic S, Torriglia A (2014) Retina: Source of the earliest biomarkers for Alzheimer’s disease? J Alzheimers Dis 40, 237–243. - PubMed
-
- London A, Benhar I, Schwartz M (2013) The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol 9, 44–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

