Dmp1 Promoter-Driven Diphtheria Toxin Receptor Transgene Expression Directs Unforeseen Effects in Multiple Tissues
- PMID: 28035954
- PMCID: PMC5297664
- DOI: 10.3390/ijms18010029
Dmp1 Promoter-Driven Diphtheria Toxin Receptor Transgene Expression Directs Unforeseen Effects in Multiple Tissues
Abstract
Mice harbouring a dentin matrix protein 1 (Dmp1) promoter-driven human diphtheria toxin (DT) receptor (HDTR) transgene (Tg) have recently been used to attain targeted ablation of osteocytes by diphtheria toxin (DT) treatment in order to define osteocyte function. Use of these Tg mice has asserted mechano- and novel paracrine regulatory osteocyte functions. To explore osteocyte roles fully, we sought to confirm the selectivity of DT effects in these transgenic mice. However, our findings revealed incomplete DT-induced osteocyte ablation, prevalent HDTR misexpression, as well as more prominent histopathological DT-induced changes in multiple organs in Tg than in wild-type (WT) littermate mice. Mechanistic evidence for DT action, via prominent regulation of phosphorylation status of elongation factor-2 (EF-2), was also found in many non-skeletal tissues in Tg mice; indicative of direct "off-target" DT action. Finally, very rapid deterioration in health and welfare status in response to DT treatment was observed in these Tg when compared to WT control mice. Together, these data lead us to conclude that alternative models for osteocyte ablation should be sought and caution be exercised when drawing conclusions from experiments using these Tg mice alone.
Keywords: bone; diphtheria toxin receptor; osteocyte.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
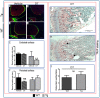
 ), viable osteocyte; shown by arrow (
), viable osteocyte; shown by arrow ( ), empty lacuna) was only observed in Tg mice treated with DT for five consecutive days (M) and only low levels (<10%) in WT and Tg mice after single DT treatment (P). Statistical comparisons: * p < 0.05 WT and Tg. Assessment of DT-induced apoptosis by TUNEL staining after single DT treatment in cortical bone of Tg (Q) and WT (R) revealed a very low number of apoptosis-positive osteocytes (arrow); arrowheads indicate negative cells. White-dotted boxes show a magnification of similar regions to better visualise the presence or lack of apoptotic cells. Negative (S) and positive (T) controls demonstrate a lack of staining in negative and many stained cells in positive control. Scale bar, 200 µm in the full overview images and 50 µm in the insets. Graphs represent the means ± SEM; ns = not significant.
), empty lacuna) was only observed in Tg mice treated with DT for five consecutive days (M) and only low levels (<10%) in WT and Tg mice after single DT treatment (P). Statistical comparisons: * p < 0.05 WT and Tg. Assessment of DT-induced apoptosis by TUNEL staining after single DT treatment in cortical bone of Tg (Q) and WT (R) revealed a very low number of apoptosis-positive osteocytes (arrow); arrowheads indicate negative cells. White-dotted boxes show a magnification of similar regions to better visualise the presence or lack of apoptotic cells. Negative (S) and positive (T) controls demonstrate a lack of staining in negative and many stained cells in positive control. Scale bar, 200 µm in the full overview images and 50 µm in the insets. Graphs represent the means ± SEM; ns = not significant.
 ), viable osteocyte; shown by arrow (
), viable osteocyte; shown by arrow ( ), empty lacuna) was only observed in Tg mice treated with DT for five consecutive days (M) and only low levels (<10%) in WT and Tg mice after single DT treatment (P). Statistical comparisons: * p < 0.05 WT and Tg. Assessment of DT-induced apoptosis by TUNEL staining after single DT treatment in cortical bone of Tg (Q) and WT (R) revealed a very low number of apoptosis-positive osteocytes (arrow); arrowheads indicate negative cells. White-dotted boxes show a magnification of similar regions to better visualise the presence or lack of apoptotic cells. Negative (S) and positive (T) controls demonstrate a lack of staining in negative and many stained cells in positive control. Scale bar, 200 µm in the full overview images and 50 µm in the insets. Graphs represent the means ± SEM; ns = not significant.
), empty lacuna) was only observed in Tg mice treated with DT for five consecutive days (M) and only low levels (<10%) in WT and Tg mice after single DT treatment (P). Statistical comparisons: * p < 0.05 WT and Tg. Assessment of DT-induced apoptosis by TUNEL staining after single DT treatment in cortical bone of Tg (Q) and WT (R) revealed a very low number of apoptosis-positive osteocytes (arrow); arrowheads indicate negative cells. White-dotted boxes show a magnification of similar regions to better visualise the presence or lack of apoptotic cells. Negative (S) and positive (T) controls demonstrate a lack of staining in negative and many stained cells in positive control. Scale bar, 200 µm in the full overview images and 50 µm in the insets. Graphs represent the means ± SEM; ns = not significant.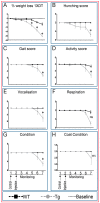

 ) as wells as osteoblasts (D, arrowhead;
) as wells as osteoblasts (D, arrowhead;  ), bone marrow cells (E, arrowhead) and around vessels (E, arrow), kidney tubules (F, arrow), and lung alveolar wall (G, arrow). Respective tissues show negative staining in WT (H–K). (L–O) are PBS negative controls. Scale bar, 200 µm.
), bone marrow cells (E, arrowhead) and around vessels (E, arrow), kidney tubules (F, arrow), and lung alveolar wall (G, arrow). Respective tissues show negative staining in WT (H–K). (L–O) are PBS negative controls. Scale bar, 200 µm.
 ) as wells as osteoblasts (D, arrowhead;
) as wells as osteoblasts (D, arrowhead;  ), bone marrow cells (E, arrowhead) and around vessels (E, arrow), kidney tubules (F, arrow), and lung alveolar wall (G, arrow). Respective tissues show negative staining in WT (H–K). (L–O) are PBS negative controls. Scale bar, 200 µm.
), bone marrow cells (E, arrowhead) and around vessels (E, arrow), kidney tubules (F, arrow), and lung alveolar wall (G, arrow). Respective tissues show negative staining in WT (H–K). (L–O) are PBS negative controls. Scale bar, 200 µm.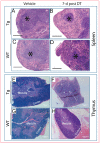

 ) as early as three days after DT treatment (B,E), before any overt osteocyte death (Figure 1J–P). Kidney shows an apparent recovery in these pathological changes seven days post DT (C,F) with increased serum calcium and phosphorous levels in treated Tg mice seven days post DT (G,H). Scale bar 200 µm. Graphs represent means ± SEM. Statistical comparisons: * p < 0.05 and ** p < 0.01 vehicle and DT treated within each genotype; (a) denotes significant differences between vehicle-treated WT and Tg mice (p < 0.05).
) as early as three days after DT treatment (B,E), before any overt osteocyte death (Figure 1J–P). Kidney shows an apparent recovery in these pathological changes seven days post DT (C,F) with increased serum calcium and phosphorous levels in treated Tg mice seven days post DT (G,H). Scale bar 200 µm. Graphs represent means ± SEM. Statistical comparisons: * p < 0.05 and ** p < 0.01 vehicle and DT treated within each genotype; (a) denotes significant differences between vehicle-treated WT and Tg mice (p < 0.05).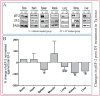
References
-
- Klein-Nulend J., van der Plas A., Semeins C.M., Ajubi N.E., Frangos J.A., Nijweide P.J., Burger E.H. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9:441–445. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

