Merosesquiterpene Congeners from the Australian Sponge Hyrtios digitatus as Potential Drug Leads for Atherosclerosis Disease
- PMID: 28036007
- PMCID: PMC5295226
- DOI: 10.3390/md15010006
Merosesquiterpene Congeners from the Australian Sponge Hyrtios digitatus as Potential Drug Leads for Atherosclerosis Disease
Abstract
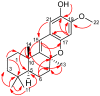
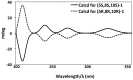
A study of the chemical constituents from the Australian Sponge Hyrtios digitatus has provided a perspective on the connection between the chemistry and biology of the puupehenones, a unique and unusual class of merosesquiterpenes. In this study, a new tetracyclic merosesquiterpene, 19-methoxy-9,15-ene-puupehenol (1) was isolated from the marine sponge Hyrtios digitatus along with the known 20-methoxy-9,15-ene-puupehenol (2). Their structures were elucidated on the basis of spectroscopic data (¹H and 13C NMR) in combination with experimental electronic circular dichroism (ECD) data. Compounds 1 and 2 are active at 1.78 μM and 3.05 μM, respectively, on Scavenger Receptor-Class B Type 1 HepG2 (SR-B1 HepG2) stable cell lines, targeting atherosclerosis disease.
Keywords: HepG2; Hyrtios digitatus; SR-B1; atherosclerosis; merosesquiterpene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- MarinLit A Database Marine Natural Products Literature. [(accessed on 30 November 2015)]. Available online: http://pubs.rsc.org/marinlit/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

