Subcellular Trafficking of Mammalian Lysosomal Proteins: An Extended View
- PMID: 28036022
- PMCID: PMC5297682
- DOI: 10.3390/ijms18010047
Subcellular Trafficking of Mammalian Lysosomal Proteins: An Extended View
Abstract
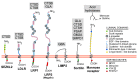
Lysosomes clear macromolecules, maintain nutrient and cholesterol homeostasis, participate in tissue repair, and in many other cellular functions. To assume these tasks, lysosomes rely on their large arsenal of acid hydrolases, transmembrane proteins and membrane-associated proteins. It is therefore imperative that, post-synthesis, these proteins are specifically recognized as lysosomal components and are correctly sorted to this organelle through the endosomes. Lysosomal transmembrane proteins contain consensus motifs in their cytosolic regions (tyrosine- or dileucine-based) that serve as sorting signals to the endosomes, whereas most lysosomal acid hydrolases acquire mannose 6-phosphate (Man-6-P) moieties that mediate binding to two membrane receptors with endosomal sorting motifs in their cytosolic tails. These tyrosine- and dileucine-based motifs are tickets for boarding in clathrin-coated carriers that transport their cargo from the trans-Golgi network and plasma membrane to the endosomes. However, increasing evidence points to additional mechanisms participating in the biogenesis of lysosomes. In some cell types, for example, there are alternatives to the Man-6-P receptors for the transport of some acid hydrolases. In addition, several "non-consensus" sorting motifs have been identified, and atypical transport routes to endolysosomes have been brought to light. These "unconventional" or "less known" transport mechanisms are the focus of this review.
Keywords: alternative receptor; lysosome; mannose 6-phosphate; sorting motif; trafficking; unconventional.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

