Sugar Allocation to Metabolic Pathways is Tightly Regulated and Affects the Virulence of Streptococcus mutans
- PMID: 28036052
- PMCID: PMC5295006
- DOI: 10.3390/genes8010011
Sugar Allocation to Metabolic Pathways is Tightly Regulated and Affects the Virulence of Streptococcus mutans
Abstract
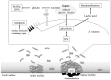
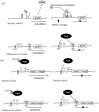
Bacteria take up and metabolize sugar as a carbohydrate source for survival. Most bacteria can utilize many sugars, including glucose, sucrose, and galactose, as well as amino sugars, such as glucosamine and N-acetylglucosamine. After entering the cytoplasm, the sugars are mainly allocated to the glycolysis pathway (energy production) and to various bacterial component biosynthesis pathways, including the cell wall, nucleic acids and amino acids. Sugars are also utilized to produce several virulence factors, such as capsule and lipoteichoic acid. Glutamine-fructose-6-phosphate aminotransferase (GlmS) and glucosamine-6-phosphate deaminase (NagB) have crucial roles in sugar distribution to the glycolysis pathway and to cell wall biosynthesis. In Streptococcus mutans, a cariogenic pathogen, the expression levels of glmS and nagB are coordinately regulated in response to the presence or absence of amino sugars. In addition, the disruption of this regulation affects the virulence of S. mutans. The expression of nagB and glmS is regulated by NagR in S. mutans, but the precise mechanism underlying glmS regulation is not clear. In Staphylococcus aureus and Bacillus subtilis, the mRNA of glmS has ribozyme activity and undergoes self-degradation at the mRNA level. However, there is no ribozyme activity region on glmS mRNA in S. mutans. In this review article, we summarize the sugar distribution, particularly the coordinated regulation of GlmS and NagB expression, and its relationship with the virulence of S. mutans.
Keywords: GlmS; NagB; Streptococcus mutans; sugar distribution; virulence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
GlmS and NagB regulate amino sugar metabolism in opposing directions and affect Streptococcus mutans virulence.PLoS One. 2012;7(3):e33382. doi: 10.1371/journal.pone.0033382. Epub 2012 Mar 16. PLoS One. 2012. PMID: 22438919 Free PMC article.
-
NagR Differentially Regulates the Expression of the glmS and nagAB Genes Required for Amino Sugar Metabolism by Streptococcus mutans.J Bacteriol. 2015 Nov;197(22):3533-44. doi: 10.1128/JB.00606-15. Epub 2015 Aug 31. J Bacteriol. 2015. PMID: 26324448 Free PMC article.
-
Amino Sugars Enhance the Competitiveness of Beneficial Commensals with Streptococcus mutans through Multiple Mechanisms.Appl Environ Microbiol. 2016 May 31;82(12):3671-82. doi: 10.1128/AEM.00637-16. Print 2016 Jun 15. Appl Environ Microbiol. 2016. PMID: 27084009 Free PMC article.
-
Xylitol: a review of its action on mutans streptococci and dental plaque--its clinical significance.Int Dent J. 1995 Feb;45(1 Suppl 1):77-92. Int Dent J. 1995. PMID: 7607748 Review.
-
The microbiology and histopathology of human root caries.Am J Dent. 1995 Dec;8(6):323-8. Am J Dent. 1995. PMID: 8695011 Review.
Cited by
-
Arginine-induced metabolomic perturbation in Streptococcus mutans.J Oral Microbiol. 2022 Jan 7;14(1):2015166. doi: 10.1080/20002297.2021.2015166. eCollection 2022. J Oral Microbiol. 2022. PMID: 35024088 Free PMC article.
-
Selective Biofilm Inhibition through Mucin-Inspired Engineering of Silk Glycopolymers.J Am Chem Soc. 2024 Dec 18;146(50):34661-34668. doi: 10.1021/jacs.4c12945. Epub 2024 Dec 9. J Am Chem Soc. 2024. PMID: 39651958 Free PMC article.
-
Use of a combined antibacterial synergy approach and the ANNOgesic tool to identify novel targets within the gene networks of multidrug-resistant Klebsiella pneumoniae.mSystems. 2024 Mar 19;9(3):e0087723. doi: 10.1128/msystems.00877-23. Epub 2024 Feb 13. mSystems. 2024. PMID: 38349171 Free PMC article.
-
An oxalate decarboxylase-like cupin domain containing protein is involved in imparting acid stress tolerance in Bacillus amyloliquefaciens MBNC.World J Microbiol Biotechnol. 2024 Jan 8;40(2):64. doi: 10.1007/s11274-023-03870-3. World J Microbiol Biotechnol. 2024. PMID: 38189984
-
Polymerase chain reaction-based identification of various serotypes of Streptococcus mutans in adults with and without dental caries.J Conserv Dent Endod. 2024 Mar;27(3):315-320. doi: 10.4103/JCDE.JCDE_312_23. Epub 2024 Mar 6. J Conserv Dent Endod. 2024. PMID: 38634021 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

