Shared and Distinct Aspects of the Sepsis Transcriptomic Response to Fecal Peritonitis and Pneumonia
- PMID: 28036233
- PMCID: PMC5549866
- DOI: 10.1164/rccm.201608-1685OC
Shared and Distinct Aspects of the Sepsis Transcriptomic Response to Fecal Peritonitis and Pneumonia
Abstract
Rationale: Heterogeneity in the septic response has hindered efforts to understand pathophysiology and develop targeted therapies. Source of infection, with different causative organisms and temporal changes, might influence this heterogeneity.
Objectives: To investigate individual and temporal variations in the transcriptomic response to sepsis due to fecal peritonitis, and to compare these with the same parameters in community-acquired pneumonia.
Methods: We performed genome-wide gene expression profiling in peripheral blood leukocytes of adult patients admitted to intensive care with sepsis due to fecal peritonitis (n = 117) or community-acquired pneumonia (n = 126), and of control subjects without sepsis (n = 10).
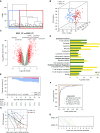
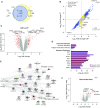
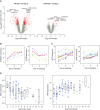
Measurements and main results: A substantial portion of the transcribed genome (18%) was differentially expressed compared with that of control subjects, independent of source of infection, with eukaryotic initiation factor 2 signaling being the most enriched canonical pathway. We identified two sepsis response signature (SRS) subgroups in fecal peritonitis associated with early mortality (P = 0.01; hazard ratio, 4.78). We defined gene sets predictive of SRS group, and serial sampling demonstrated that subgroup membership is dynamic during intensive care unit admission. We found that SRS is the major predictor of transcriptomic variation; a small number of genes (n = 263) were differentially regulated according to the source of infection, enriched for IFN signaling and antigen presentation. We define temporal changes in gene expression from disease onset involving phagosome formation as well as natural killer cell and IL-3 signaling.
Conclusions: The majority of the sepsis transcriptomic response is independent of the source of infection and includes signatures reflecting immune response state and prognosis. A modest number of genes show evidence of specificity. Our findings highlight opportunities for patient stratification and precision medicine in sepsis.
Keywords: gene expression; immune; patient stratification; septic.
Figures

Comment in
-
Leveraging Transcriptomics to Disentangle Sepsis Heterogeneity.Am J Respir Crit Care Med. 2017 Aug 1;196(3):258-260. doi: 10.1164/rccm.201701-0143ED. Am J Respir Crit Care Med. 2017. PMID: 28762788 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

