MicroRNA 603 acts as a tumor suppressor and inhibits triple-negative breast cancer tumorigenesis by targeting elongation factor 2 kinase
- PMID: 28036267
- PMCID: PMC5355293
- DOI: 10.18632/oncotarget.14264
MicroRNA 603 acts as a tumor suppressor and inhibits triple-negative breast cancer tumorigenesis by targeting elongation factor 2 kinase
Abstract
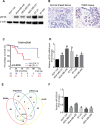
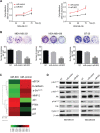
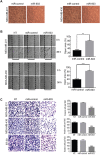
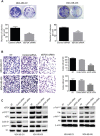
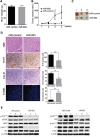
Triple negative breast cancer (TNBC) is an aggressive type of breast cancer characterized by the absence of defined molecular targets, including estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and is associated with high rates of relapse and distant metastasis despite surgery and adjuvant chemotherapy. The lack of effective targeted therapies for TNBC represents an unmet therapeutic challenge. Eukaryotic elongation factor 2 kinase (eEF2K) is an atypical calcium/calmodulin-dependent serine/threonine kinase that promotes TNBC tumorigenesis, progression, and drug resistance, representing a potential novel molecular target. However, the mechanisms regulating eEF2K expression are unknown. Here, we report that eEF2K protein expression is highly up-regulated in TNBC cells and patient tumors and it is associated with poor patient survival and clinical outcome. We found that loss/reduced expression of miR-603 leads to eEF2K overexpression in TNBC cell lines. Its expression results in inhibition of eEF2K by directly targeting the 3-UTR and the inhibition of tumor cell growth, migration and invasion in TNBC. In vivo therapeutic gene delivery of miR-603 into TNBC xenograft mouse models by systemic administration of miR-603-nanoparticles led to a significant inhibition of eEF2K expression and tumor growth, which was associated with decreased activity of the downstream targets of eEF2K, including Src, Akt, cyclin D1 and c-myc. Our findings suggest that miR-603 functions as a tumor suppressor and loss of miR-603 expression leads to increase in eEF2K expression and contributes to the growth, invasion, and progression of TNBC. Taken together, our data suggest that miR-603-based gene therapy is a potential strategy against TNBC.
Keywords: eEF2K; liposomes; miR-603; nanoparticles; triple negative breast cancer.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer. 2013;49:1374–1403. - PubMed
-
- DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. - PubMed
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
-
- Dawood S. Triple-negative breast cancer: epidemiology and management options. Drugs. 2010;70:2247–2258. - PubMed
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. The New England journal of medicine. 2010;363:1938–1948. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

