Polyunsaturated fatty acids ameliorate aging via redox-telomere-antioncogene axis
- PMID: 28038469
- PMCID: PMC5352322
- DOI: 10.18632/oncotarget.14236
Polyunsaturated fatty acids ameliorate aging via redox-telomere-antioncogene axis
Abstract
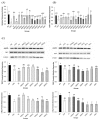
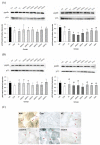
Polyunsaturated fatty acids (PUFA), a group of nourishing and health-promoting nutrients, ameliorate age-related chronic diseases. However, how PUFA especially n-3 PUFA exert anti-aging functions remains poorly understood. Here we link fish oil, docosahexaenoic acid (DHA) and arachidonic acid (AA) to the aging etiology via a redox-telomere-antioncogene axis based on D-galactose-induced aging mice. Both fish oil and PUFA enhanced hepatic superoxide dismutase (SOD) and catalase activities and cardiac SOD activities within the range of 18%-46%, 26%-65% and 19%-58%, respectively, whereas reduced cerebral monoamine oxidase activity, plasma F2-isoprostane level and cerebral lipid peroxidation level by 56%-90%, 20%-79% and 16%-54%, respectively. Thus, PUFA improve the in vivo redox and oxidative stress induced aging process, which however does not exhibit a dose-dependent manner. Notably, both PUFA and fish oil effectively inactivated testicular telomerase and inhibited c-Myc-mediated telomerase reverse transcriptase expression, whereas n-3 PUFA rather than n-6 PUFA protected liver and testes against telomere shortening within the range of 13%-25% and 25%-27%, respectively. Therefore, n-3 PUFA may be better at inhibiting the DNA damage induced aging process. Surprisingly, only DHA significantly suppressed cellular senescence pathway evidenced by testicular antioncogene p16 and p53 expression. This work provides evident support for the crosstalk between PUFA especially n-3 PUFA and the aging process via maintaining the in vivo redox homeostasis, rescuing age-related telomere attrition and down-regulating the antioncogene expression.
Keywords: Gerotarget; anti-aging; antioncogene; polyunsaturated fatty acids; redox; telomere protection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sanchez JR, Milton SL, Corbit KC, Buffenstein R. Multifactorial processes to slowing the biological clock: Insights from a comparative approach. Exp Gerontol. 2015;71:27–37. - PubMed
-
- Gambino V, De Michele G, Venezia O, Migliaccio P, Dall’Olio V, Bernard L, Minardi SP, Fazia MAD, Bartoli D, Servillo G, Alcalay M, Luzi L, et al. Oxidative stress activates a specific p53 transcriptional response that regulates cellular senescence and aging. Aging Cell. 2013;12:435–445. - PMC - PubMed
-
- Budni J, Pacheco R, S Da Silva, Garcez ML, Mina F, Bellettini-Santos T, de Medeiros J, Voss BC, Steckert AV, Valvassori SDS, Quevedo J. Oral administration of d-galactose induces cognitive impairments and oxidative damage in rats. Behav Brain Res. 2016;302:35–43. - PubMed
-
- Ho SC, Liu JH, Wu RY. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology. 2003;4:15–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

