Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics
- PMID: 28039063
- PMCID: PMC5455143
- DOI: 10.1016/j.actbio.2016.12.049
Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics
Abstract
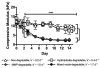
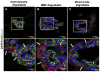
Radiation therapy for head and neck cancers leads to permanent xerostomia due to the loss of secretory acinar cells in the salivary glands. Regenerative treatments utilizing primary submandibular gland (SMG) cells show modest improvements in salivary secretory function, but there is limited evidence of salivary gland regeneration. We have recently shown that poly(ethylene glycol) (PEG) hydrogels can support the survival and proliferation of SMG cells as multicellular spheres in vitro. To further develop this approach for cell-based salivary gland regeneration, we have investigated how different modes of PEG hydrogel degradation affect the proliferation, cell-specific gene expression, and epithelial morphology within encapsulated salivary gland spheres. Comparison of non-degradable, hydrolytically-degradable, matrix metalloproteinase (MMP)-degradable, and mixed mode-degradable hydrogels showed that hydrogel degradation by any mechanism is required for significant proliferation of encapsulated cells. The expression of acinar phenotypic markers Aqp5 and Nkcc1 was increased in hydrogels that are MMP-degradable compared with other hydrogel compositions. However, expression of secretory acinar proteins Mist1 and Pip was not maintained to the same extent as phenotypic markers, suggesting changes in cell function upon encapsulation. Nevertheless, MMP- and mixed mode-degradability promoted organization of polarized cell types forming tight junctions and expression of the basement membrane proteins laminin and collagen IV within encapsulated SMG spheres. This work demonstrates that cellularly remodeled hydrogels can promote proliferation and gland-like organization by encapsulated salivary gland cells as well as maintenance of acinar cell characteristics required for regenerative approaches. Investigation is required to identify approaches to further enhance acinar secretory properties.
Statement of significance: Regenerative strategies to replace damaged salivary glands require the function and organization of acinar cells. Hydrogel-based approaches have shown promise to control cell function and phenotype. However, little is known about how specific parameters, such as the mechanism of hydrogel degradation (e.g., hydrolytic or enzymatic), influence the viability, proliferation, organization, and phenotype of salivary gland cells. In this work, it is shown that hydrogel-encapsulated primary salivary gland cell proliferation is dependent upon hydrogel degradation. Hydrogels crosslinked with enzymatically degradable peptides promoted the expression of critical acinar cell markers, which are typically downregulated in primary cultures. Furthermore, salivary gland cells encapsulated in enzymatically- but not hydrolytically-degradable hydrogels displayed highly organized and polarized salivary gland cell markers, which mimics characteristics found in native gland tissue. In sum, results indicate that salivary gland cells respond to cellularly remodeled hydrogels, resulting in self-assembly and organization akin to acini substructures of the salivary gland.
Keywords: Acinar cells; Degradation; Hydrogel; Poly(ethylene glycol); Salivary gland.
Copyright © 2016 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures






References
-
- Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, Dutilh J, Fulton JS, Jankovic L, Lopes NN, Mello AL, Muniz LV, Murdoch-Kinch CA, Nair RG, Napenas JJ, Nogueira-Rodrigues A, Saunders D, Stirling B, Von Bultzingslowen I, Weikel DS, Elting LS, Spijkervet FK, Brennan MT, Salivary S. Gland Hypofunction/Xerostomia, G. Oral Care Study, O. Multinational Association of Supportive Care in Cancer /International Society of Oral, A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18:1061–1079. - PubMed
-
- Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. - PubMed
-
- Cotrim AP, Sowers A, Mitchell JB, Baum BJ. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther. 2007;15:2101–2106. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

