Everolimus-eluting stents stabilize plaque inflammation in vivo: assessment by intravascular fluorescence molecular imaging
- PMID: 28039209
- PMCID: PMC5837269
- DOI: 10.1093/ehjci/jew228
Everolimus-eluting stents stabilize plaque inflammation in vivo: assessment by intravascular fluorescence molecular imaging
Abstract
Aims: Inflammation drives atherosclerosis complications and is a promising therapeutic target for plaque stabilization. At present, it is unknown whether local stenting approaches can stabilize plaque inflammation in vivo. Here, we investigate whether everolimus-eluting stents (EES) can locally suppress plaque inflammatory protease activity in vivo using intravascular near-infrared fluorescence (NIRF) molecular imaging.
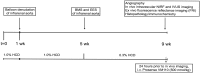
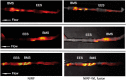
Methods and results: Balloon-injured, hyperlipidaemic rabbits with atherosclerosis received non-overlapping EES and bare metal stents (BMS) placement into the infrarenal aorta (n = 7 EES, n = 7 BMS, 3.5 mm diameter x 12 mm length). Four weeks later, rabbits received an injection of the cysteine protease-activatable NIRF imaging agent Prosense VM110. Twenty-four hours later, co-registered intravascular 2D NIRF, X-ray angiography and intravascular ultrasound imaging were performed. In vivo EES-stented plaques contained substantially reduced NIRF inflammatory protease activity compared with untreated plaques and BMS-stented plaques (P = 0.006). Ex vivo macroscopic NIRF imaging of plaque protease activity corroborated the in vivo results (P = 0.003). Histopathology analyses revealed that EES-treated plaques showed reduced neointimal and medial arterial macrophage and cathepsin B expression compared with unstented and BMS-treated plaques.
Conclusions: EES-stenting stabilizes plaque inflammation as assessed by translational intravascular NIRF molecular imaging in vivo. These data further support that EES may provide a local approach for stabilizing inflamed plaques.
Keywords: atherosclerosis; everolimus; fluorescence imaging; inflammation; molecular imaging; stent.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2016. For permissions, please email: journals.permissions@oup.com.
Figures




Comment in
-
Shedding light on inflammation.Eur Heart J Cardiovasc Imaging. 2017 May 1;18(5):519-520. doi: 10.1093/ehjci/jew297. Eur Heart J Cardiovasc Imaging. 2017. PMID: 28065913 Free PMC article. No abstract available.
References
-
- Libby P, Ridker PM, Hansson GK.. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–25. - PubMed
-
- Wykrzykowska JJ, Diletti R, Gutierrez-Chico JL, van Geuns RJ, van der Giessen WJ, Ramcharitar S. et al. Plaque sealing and passivation with a mechanical self-expanding low outward force nitinol vShield device for the treatment of IVUS and OCT-derived thin cap fibroatheromas (TCFAs) in native coronary arteries: report of the pilot study vShield Evaluated at Cardiac hospital in Rotterdam for Investigation and Treatment of TCFA (SECRITT). EuroIntervention 2012;8:945–54. - PubMed
-
- Verheye S, Martinet W, Kockx MM, Knaapen MW, Salu K, Timmermans JP. et al. Selective clearance of macrophages in atherosclerotic plaques by autophagy. J Am Coll Cardiol 2007;49:706–15. - PubMed
-
- Van Dyck CJ, Hoymans VY, Bult H, Fransen E, Haine S, Bosmans JM. et al. Resolute and Xience V polymer-based drug-eluting stents compared in an atherosclerotic rabbit double injury model. Catheter Cardiovasc Interv 2013;81:E259–68. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

