Variants in WFS1 and Other Mendelian Deafness Genes Are Associated with Cisplatin-Associated Ototoxicity
- PMID: 28039263
- PMCID: PMC5493516
- DOI: 10.1158/1078-0432.CCR-16-2809
Variants in WFS1 and Other Mendelian Deafness Genes Are Associated with Cisplatin-Associated Ototoxicity
Abstract
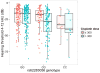
Purpose: Cisplatin is one of the most commonly used chemotherapy drugs worldwide and one of the most ototoxic. We sought to identify genetic variants that modulate cisplatin-associated ototoxicity (CAO).Experimental Design: We performed a genome-wide association study (GWAS) of CAO using quantitative audiometry (4-12 kHz) in 511 testicular cancer survivors of European genetic ancestry. We performed polygenic modeling and functional analyses using a variety of publicly available databases. We used an electronic health record cohort to replicate our top mechanistic finding.Results: One SNP, rs62283056, in the first intron of Mendelian deafness gene WFS1 (wolframin ER transmembrane glycoprotein) and an expression quantitative trait locus (eQTL) for WFS1 met genome-wide significance for association with CAO (P = 1.4 × 10-8). A significant interaction between cumulative cisplatin dose and rs62283056 genotype was evident, indicating that higher cisplatin doses exacerbate hearing loss in patients with the minor allele (P = 0.035). The association between decreased WFS1 expression and hearing loss was replicated in an independent BioVU cohort (n = 18,620 patients, Bonferroni adjusted P < 0.05). Beyond this top signal, we show CAO is a polygenic trait and that SNPs in and near 84 known Mendelian deafness genes are significantly enriched for low P values in the GWAS (P = 0.048).Conclusions: We show for the first time the role of WFS1 in CAO and document a statistically significant interaction between increasing cumulative cisplatin dose and rs62283056 genotype. Our clinical translational results demonstrate that pretherapy patient genotyping to minimize ototoxicity could be useful when deciding between cisplatin-based chemotherapy regimens of comparable efficacy with different cumulative doses. Clin Cancer Res; 23(13); 3325-33. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

