Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol
- PMID: 28039295
- PMCID: PMC5223707
- DOI: 10.1136/bmjopen-2016-013904
Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol
Abstract
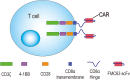
Introduction: There is no curative treatment available for patients with chemotherapy relapsed or refractory CD19+ B cells-derived acute lymphoblastic leukaemia (r/r B-ALL). Although CD19-targeting second-generation (2nd-G) chimeric antigen receptor (CAR)-modified T cells carrying CD28 or 4-1BB domains have demonstrated potency in patients with advanced B-ALL, these 2 signalling domains endow CAR-T cells with different and complementary functional properties. Preclinical results have shown that third-generation (3rd-G) CAR-T cells combining 4-1BB and CD28 signalling domains have superior activation and proliferation capacity compared with 2nd-G CAR-T cells carrying CD28 domain. The aim of the current study is therefore to investigate the safety and efficacy of 3rd-G CAR-T cells in adults with r/r B-ALL.
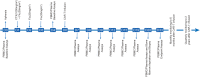
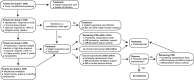
Methods and analysis: This study is a phase I clinical trial for patients with r/r B-ALL to test the safety and preliminary efficacy of 3rd-G CAR-T cells. Before receiving lymphodepleting conditioning regimen, the peripheral blood mononuclear cells from eligible patients will be leukapheresed, and the T cells will be purified, activated, transduced and expanded ex vivo. On day 6 in the protocol, a single dose of 1 million CAR-T cells per kg will be administrated intravenously. The phenotypes of infused CAR-T cells, copy number of CAR transgene and plasma cytokines will be assayed for 2 years after CAR-T infusion using flow cytometry, real-time quantitative PCR and cytometric bead array, respectively. Moreover, several predictive plasma cytokines including interferon-γ, interleukin (IL)-6, IL-8, Soluble Interleukin (sIL)-2R-α, solubleglycoprotein (sgp)130, sIL-6R, Monocyte chemoattractant protein (MCP1), Macrophage inflammatory protein (MIP1)-α, MIP1-β and Granulocyte-macrophage colony-stimulating factor (GM-CSF), which are highly associated with severe cytokine release syndrome (CRS), will be used to forecast CRS to allow doing earlier intervention, and CRS will be managed based on a revised CRS grading system. In addition, patients with grade 3 or 4 neurotoxicities or persistent B-cell aplasia will be treated with dexamethasone (10 mg intravenously every 6 hours) or IgG, respectively. Descriptive and analytical analyses will be performed.
Ethics and dissemination: Ethical approval for the study was granted on 10 July 2014 (YLJS-2014-7-10). Written informed consent will be taken from all participants. The results of the study will be reported, through peer-reviewed journals, conference presentations and an internal organisational report.
Trial registration number: NCT02186860.
Keywords: IMMUNOLOGY; Third-generation; acute lymphoblastic leukemia; chimeric antigen receptor.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
