Dissecting the Influence of Two Structural Substituents on the Differential Neurotoxic Effects of Acute Methamphetamine and Mephedrone Treatment on Dopamine Nerve Endings with the Use of 4-Methylmethamphetamine and Methcathinone
- PMID: 28039330
- PMCID: PMC5325074
- DOI: 10.1124/jpet.116.237768
Dissecting the Influence of Two Structural Substituents on the Differential Neurotoxic Effects of Acute Methamphetamine and Mephedrone Treatment on Dopamine Nerve Endings with the Use of 4-Methylmethamphetamine and Methcathinone
Abstract
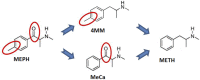
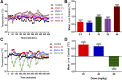
Mephedrone (MEPH) is a β-ketoamphetamine stimulant drug of abuse that is often a constituent of illicit bath salts formulations. Although MEPH bears remarkable similarities to methamphetamine (METH) in terms of chemical structure, as well as its neurochemical and behavioral effects, it has been shown to have a reduced neurotoxic profile compared with METH. The addition of a β-keto moiety and a 4-methyl ring substituent to METH yields MEPH, and a loss of direct neurotoxic potential. In the present study, two analogs of METH, methcathinone (MeCa) and 4-methylmethamphetamine (4MM), were assessed for their effects on mouse dopamine (DA) nerve endings to determine the relative contribution of each individual moiety to the loss of direct neurotoxicity in MEPH. Both MeCa and 4MM caused significant alterations in core body temperature as well as locomotor activity and stereotypy, but 4MM was found to elicit minimal dopaminergic toxicity only at the highest dose. By contrast, MeCa caused significant reductions in all markers of DA nerve-ending damage over a range of doses. These results lead to the conclusion that ring substitution at the 4-position profoundly reduces the neurotoxicity of METH, whereas the β-keto group has much less influence on this property. Although the mechanism(s) by which the 4-methyl substituent reduces METH-induced neurotoxicity remains unclear, it is speculated that this effect is mediated by a loss of DA-releasing action in MEPH and 4MM at the synaptic vesicle monoamine transporter, an effect that is thought to be critical for METH-induced neurotoxicity.
Copyright © 2017 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Ali SF, Newport GD, Holson RR, Slikker W, Jr, Bowyer JF. (1994) Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res 658:33–38. - PubMed
-
- Ali SF, Newport GD, Slikker W., Jr (1996) Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Ann N Y Acad Sci 801:187–198. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

