Extracellular vesicles of the blood-brain barrier: Role in the HIV-1 associated amyloid beta pathology
- PMID: 28040512
- PMCID: PMC5315639
- DOI: 10.1016/j.mcn.2016.12.006
Extracellular vesicles of the blood-brain barrier: Role in the HIV-1 associated amyloid beta pathology
Abstract
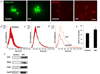
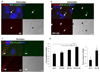
HIV-infected brains are characterized by increased amyloid beta (Aβ) deposition. It is believed that the blood-brain barrier (BBB) is critical for Aβ homeostasis and contributes to Aβ accumulation in the brain. Extracellular vesicles (ECV), like exosomes, recently gained a lot of attention as potentially playing a significant role in Aβ pathology. In addition, HIV-1 hijacks the exosomal pathway for budding and release. Therefore, we investigated the involvement of BBB-derived ECV in the HIV-1-induced Aβ pathology in the brain. Our results indicate that HIV-1 increases ECV release from brain endothelial cells as well as elevates their Aβ cargo when compared to controls. Interestingly, brain endothelial cell-derived ECV transferred Aβ to astrocytes and pericytes. Infusion of brain endothelial ECV carrying fluorescent Aβ into the internal carotid artery of mice resulted in Aβ fluorescence associated with brain microvessels and in the brain parenchyma. These results suggest that ECV carrying Aβ can be successfully transferred across the BBB into the brain. Based on these observations, we conclude that HIV-1 facilitates the shedding of brain endothelial ECV carrying Aβ; a process that may increase Aβ exposure of cells of neurovascular unit, and contribute to amyloid deposition in HIV-infected brain.
Keywords: Amyloid beta; Blood-brain barrier; Extracellular vesicles; HIV-1.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

