Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice
- PMID: 28040513
- PMCID: PMC7113847
- DOI: 10.1016/j.antiviral.2016.12.019
Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice
Abstract
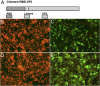
Middle East respiratory syndrome coronavirus (MERS-CoV) has continued spreading since its emergence in 2012 with a mortality rate of 35.6%, and is a potential pandemic threat. Prophylactics and therapies are urgently needed to address this public health problem. We report here the efficacy of a vaccine consisting of chimeric virus-like particles (VLP) expressing the receptor binding domain (RBD) of MERS-CoV. In this study, a fusion of the canine parvovirus (CPV) VP2 structural protein gene with the RBD of MERS-CoV can self-assemble into chimeric, spherical VLP (sVLP). sVLP retained certain parvovirus characteristics, such as the ability to agglutinate pig erythrocytes, and structural morphology similar to CPV virions. Immunization with sVLP induced RBD-specific humoral and cellular immune responses in mice. sVLP-specific antisera from these animals were able to prevent pseudotyped MERS-CoV entry into susceptible cells, with neutralizing antibody titers reaching 1: 320. IFN-γ, IL-4 and IL-2 secreting cells induced by the RBD were detected in the splenocytes of vaccinated mice by ELISpot. Furthermore, mice inoculated with sVLP or an adjuvanted sVLP vaccine elicited T-helper 1 (Th1) and T-helper 2 (Th2) cell-mediated immunity. Our study demonstrates that sVLP displaying the RBD of MERS-CoV are promising prophylactic candidates against MERS-CoV in a potential outbreak situation.
Keywords: CPV; Immune response; Middle East respiratory syndrome coronavirus; Receptor binding domain; Virus-like particles.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants.J Virol. 2016 Dec 16;91(1):e01651-16. doi: 10.1128/JVI.01651-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795425 Free PMC article.
-
MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular imminity in rhesus macaques.Oncotarget. 2017 Feb 21;8(8):12686-12694. doi: 10.18632/oncotarget.8475. Oncotarget. 2017. PMID: 27050368 Free PMC article.
-
Optimization of antigen dose for a receptor-binding domain-based subunit vaccine against MERS coronavirus.Hum Vaccin Immunother. 2015;11(5):1244-50. doi: 10.1080/21645515.2015.1021527. Hum Vaccin Immunother. 2015. PMID: 25874632 Free PMC article.
-
Prospects for a MERS-CoV spike vaccine.Expert Rev Vaccines. 2018 Aug;17(8):677-686. doi: 10.1080/14760584.2018.1506702. Epub 2018 Aug 9. Expert Rev Vaccines. 2018. PMID: 30058403 Free PMC article. Review.
-
Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain.Viruses. 2019 Jan 14;11(1):60. doi: 10.3390/v11010060. Viruses. 2019. PMID: 30646569 Free PMC article. Review.
Cited by
-
A Recombinant Influenza A/H1N1 Carrying A Short Immunogenic Peptide of MERS-CoV as Bivalent Vaccine in BALB/c Mice.Pathogens. 2019 Dec 2;8(4):281. doi: 10.3390/pathogens8040281. Pathogens. 2019. PMID: 31810359 Free PMC article.
-
Nanotechnology of inhalable vaccines for enhancing mucosal immunity.Drug Deliv Transl Res. 2024 Mar;14(3):597-620. doi: 10.1007/s13346-023-01431-7. Epub 2023 Sep 25. Drug Deliv Transl Res. 2024. PMID: 37747597 Review.
-
Characterization of the Immune Response of MERS-CoV Vaccine Candidates Derived from Two Different Vectors in Mice.Viruses. 2020 Jan 20;12(1):125. doi: 10.3390/v12010125. Viruses. 2020. PMID: 31968702 Free PMC article.
-
MS2 Virus-like Particles as a Versatile Peptide Presentation Platform: Insights into the Deterministic Abilities for Accommodating Heterologous Peptide Lengths.ACS Synth Biol. 2023 Dec 15;12(12):3704-3715. doi: 10.1021/acssynbio.3c00503. Epub 2023 Nov 9. ACS Synth Biol. 2023. PMID: 37946498 Free PMC article.
-
SARS-CoV-2-Specific Vaccine Candidates; the Contribution of Structural Vaccinology.Vaccines (Basel). 2022 Feb 3;10(2):236. doi: 10.3390/vaccines10020236. Vaccines (Basel). 2022. PMID: 35214693 Free PMC article. Review.
References
-
- Barcena J., Blanco E. Design of novel vaccines based on virus-like particles or chimeric virions. Subcell. Biochem. 2013;68:631–665. - PubMed
-
- Casal J.I., Rueda P., Hurtado A. Parvovirus-like particles as vaccine vectors. Methods. 1999;19(1):174–186. - PubMed
-
- Clinical Trials.gov . 2016. Phase I, Open Label Dose Ranging Safety Study of GLS-5300 in Healthy Volunteers.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

