Quantifying the extent to which index event biases influence large genetic association studies
- PMID: 28040731
- PMCID: PMC5661557
- DOI: 10.1093/hmg/ddw433
Quantifying the extent to which index event biases influence large genetic association studies
Abstract
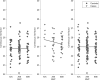
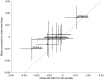
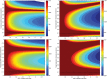
As genetic association studies increase in size to 100 000s of individuals, subtle biases may influence conclusions. One possible bias is 'index event bias' (IEB) that appears due to the stratification by, or enrichment for, disease status when testing associations between genetic variants and a disease-associated trait. We aimed to test the extent to which IEB influences some known trait associations in a range of study designs and provide a statistical framework for assessing future associations. Analyzing data from 113 203 non-diabetic UK Biobank participants, we observed three (near TCF7L2, CDKN2AB and CDKAL1) overestimated (body mass index (BMI) decreasing) and one (near MTNR1B) underestimated (BMI increasing) associations among 11 type 2 diabetes risk alleles (at P < 0.05). IEB became even stronger when we tested a type 2 diabetes genetic risk score composed of these 11 variants (-0.010 standard deviations BMI per allele, P = 5 × 10- 4), which was confirmed in four additional independent studies. Similar results emerged when examining the effect of blood pressure increasing alleles on BMI in normotensive UK Biobank samples. Furthermore, we demonstrated that, under realistic scenarios, common disease alleles would become associated at P < 5 × 10- 8 with disease-related traits through IEB alone, if disease prevalence in the sample differs appreciably from the background population prevalence. For example, some hypertension and type 2 diabetes alleles will be associated with BMI in sample sizes of >500 000 if the prevalence of those diseases differs by >10% from the background population. In conclusion, IEB may result in false positive or negative genetic associations in very large studies stratified or strongly enriched for/against disease cases.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- HHSN268201100012C/HL/NHLBI NIH HHS/United States
- HHSN268201100009I/HL/NHLBI NIH HHS/United States
- G0500070/MRC_/Medical Research Council/United Kingdom
- R01 HL086694/HL/NHLBI NIH HHS/United States
- WT097835MF/WT_/Wellcome Trust/United Kingdom
- U01 HG004402/HG/NHGRI NIH HHS/United States
- HHSN268201100005I/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- G0601261/MRC_/Medical Research Council/United Kingdom
- UL1 RR025005/RR/NCRR NIH HHS/United States
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- HHSN268201100005G/HL/NHLBI NIH HHS/United States
- HHSN268201100008I/HL/NHLBI NIH HHS/United States
- MC_QA137853/MRC_/Medical Research Council/United Kingdom
- R01 HL059367/HL/NHLBI NIH HHS/United States
- MR/M005070/1/MRC_/Medical Research Council/United Kingdom
- 102820/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- T32 HL007779/HL/NHLBI NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- MR/M005070/1/MRC_/Medical Research Council/United Kingdom
- CZD/16/6/4/CSO_/Chief Scientist Office/United Kingdom
- MC_PC_U127561128/MRC_/Medical Research Council/United Kingdom
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

