Oncogenic regulation of tumor metabolic reprogramming
- PMID: 28040803
- PMCID: PMC5308762
- DOI: 10.18632/oncotarget.10911
Oncogenic regulation of tumor metabolic reprogramming
Abstract
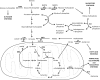
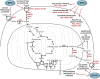
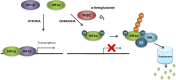
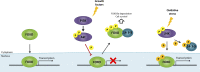
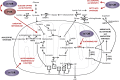
Development of malignancy is accompanied by a complete metabolic reprogramming closely related to the acquisition of most of cancer hallmarks. In fact, key oncogenic pathways converge to adapt the metabolism of carbohydrates, proteins, lipids and nucleic acids to the dynamic tumor microenvironment, conferring a selective advantage to cancer cells. Therefore, metabolic properties of tumor cells are significantly different from those of non-transformed cells. In addition, tumor metabolic reprogramming is linked to drug resistance in cancer treatment. Accordingly, metabolic adaptations are specific vulnerabilities that can be used in different therapeutic approaches for cancer therapy. In this review, we discuss the dysregulation of the main metabolic pathways that enable cell transformation and its association with oncogenic signaling pathways, focusing on the effects of c-MYC, hypoxia inducible factor 1 (HIF1), phosphoinositide-3-kinase (PI3K), and the mechanistic target of rapamycin (mTOR) on cancer cell metabolism. Elucidating these connections is of crucial importance to identify new targets and develop selective cancer treatments that improve response to therapy and overcome the emerging resistance to chemotherapeutics.
Keywords: HIF; MYC; PI3K; mTOR; metabolic reprogramming.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

