Unique cellular and humoral immunogenicity profiles generated by aerosol, intranasal, or parenteral vaccination in rhesus macaques
- PMID: 28041780
- PMCID: PMC5241230
- DOI: 10.1016/j.vaccine.2016.12.008
Unique cellular and humoral immunogenicity profiles generated by aerosol, intranasal, or parenteral vaccination in rhesus macaques
Abstract
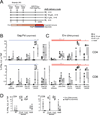
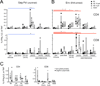
Respiratory mucosa immunization is capable of eliciting both local and distal mucosal immune responses; it is a potentially powerful yet largely unused modality for vaccination against respiratory diseases. Targeting the lower versus upper airways by aerosol delivery alters the immunogenicity profile of a vaccine, although the full extent of this impact is not well characterized. We set out to define the cellular and humoral response profiles elicited by immunization via intranasal, small aerosol droplets, and large aerosol droplets. We compared responses following adenovirus-vectored vaccination by these routes in macaques, either for the generation of primary immune responses or for the boosting of previously primed systemic responses. Aerosol delivery (4 or 10μm diameter droplets, addressing lower or upper airways, respectively) generated the highest magnitude lung CD4 and CD8 T-cell responses, reaching 10-30% vaccine-specific levels in bronchoalveolar lavage cells. In contrast, intranasal delivery was less immunogenic with >10-fold lower peak lung T-cell responses. Systemic (blood) T-cell responses were only observed following 4μm aerosol (and parenteral) immunization, while all delivery routes elicited similar humoral responses. These data demonstrate distinct immune response profiles with each respiratory tract vaccination modality and suggest that small droplet aerosol offers several immunological advantages over other respiratory routes.
Keywords: Aerosol; Immunogenicity; Nasal; Rhesus macaque; Vaccine delivery route.
Published by Elsevier Ltd.
Conflict of interest statement
Conflict of Interest. The authors declare no conflict of interest.
Figures


References
-
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nature medicine. 2005;11:S45–S53. - PubMed
-
- Nardelli-Haefliger D, Lurati F, Wirthner D, Spertini F, Schiller JT, Lowy DR, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005;23:3634–3641. - PubMed
-
- Revaz S, Dudler J. [Clinical manifestations of gout] Revue medicale suisse. 2007;3:728–730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

