The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn
- PMID: 28041852
- PMCID: PMC5236062
- DOI: 10.1016/j.cell.2016.12.010
The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn
Abstract
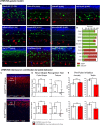
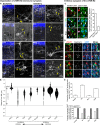
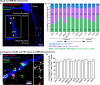
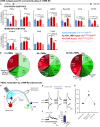
The deep dorsal horn is a poorly characterized spinal cord region implicated in processing low-threshold mechanoreceptor (LTMR) information. We report an array of mouse genetic tools for defining neuronal components and functions of the dorsal horn LTMR-recipient zone (LTMR-RZ), a role for LTMR-RZ processing in tactile perception, and the basic logic of LTMR-RZ organization. We found an unexpectedly high degree of neuronal diversity in the LTMR-RZ: seven excitatory and four inhibitory subtypes of interneurons exhibiting unique morphological, physiological, and synaptic properties. Remarkably, LTMRs form synapses on between four and 11 LTMR-RZ interneuron subtypes, while each LTMR-RZ interneuron subtype samples inputs from at least one to three LTMR classes, as well as spinal cord interneurons and corticospinal neurons. Thus, the LTMR-RZ is a somatosensory processing region endowed with a neuronal complexity that rivals the retina and functions to pattern the activity of ascending touch pathways that underlie tactile perception.
Keywords: low-threshold mechanoreceptors; mouse molecular genetics; somatosensation; spinal cord dorsal horn; spinal cord interneurons; synaptic connectivity.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Sensory systems: Sensational organization in the dorsal horn.Nat Rev Neurosci. 2017 Mar;18(3):128. doi: 10.1038/nrn.2017.9. Epub 2017 Jan 27. Nat Rev Neurosci. 2017. PMID: 28127034 No abstract available.
References
MeSH terms
Grants and funding
- S10 RR028832/RR/NCRR NIH HHS/United States
- R35 NS097344/NS/NINDS NIH HHS/United States
- P30 NS072030/NS/NINDS NIH HHS/United States
- P30 DA035756/DA/NIDA NIH HHS/United States
- U54 HD090255/HD/NICHD NIH HHS/United States
- R01 DA034022/DA/NIDA NIH HHS/United States
- BB/J000620/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- S10 RR031531/RR/NCRR NIH HHS/United States
- F32 NS077836/NS/NINDS NIH HHS/United States
- R21 DA023643/DA/NIDA NIH HHS/United States
- 102645/WT_/Wellcome Trust/United Kingdom
- P01 NS079419/NS/NINDS NIH HHS/United States
- T32 NS007292/NS/NINDS NIH HHS/United States
- U01 MH105949/MH/NIMH NIH HHS/United States
- R01 DE022750/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

