Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model
- PMID: 28042326
- PMCID: PMC5196895
- DOI: 10.7150/thno.17133
Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model
Abstract
Objectives: Osteoarthritis (OA) is the most common joint disease throughout the world. Exosomes derived from miR-140-5p-overexpressing synovial mesenchymal stem cells (SMSC-140s) may be effective in treating OA. We hypothesized that exosomes derived from SMSC-140 (SMSC-140-Exos) would enhance the proliferation and migration abilities of articular chondrocytes (ACs) without harming extracellular matrix (ECM) secretion.
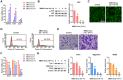
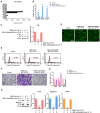
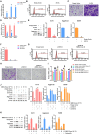
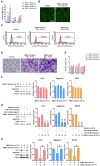
Methods: SMSCs were transfected with or without miR-140-5p. Exosomes derived from SMSCs or SMSC-140s (SMSC-Exos or SMSC-140-Exos) were isolated and identified. Proliferation, migration and ECM secretion were measured in vitro and compared between groups. The mechanism involving alternative Wnt signalling and activation of Yes-associated protein (YAP) was investigated using lentivirus, oligonucleotides or chemical drugs. The preventative effect of exosomes in vivo was measured using Safranin-O and Fast green staining and immunohistochemical staining.
Results: Wnt5a and Wnt5b carried by exosomes activated YAP via the alternative Wnt signalling pathway and enhanced proliferation and migration of chondrocytes with the side-effect of significantly decreasing ECM secretion. Highly-expressed miR-140-5p blocked this side-effect via RalA. SMSC-140-Exos enhanced the proliferation and migration of ACs without damaging ECM secretion in vitro, while in vivo, SMSC-140-Exos successfully prevented OA in a rat model.
Conclusions: These findings highlight the promising potential of SMSC-140-Exos in preventing OA. We first found a potential source of exosomes and studied their merits and shortcomings. Based on our understanding of the molecular mechanism, we overcame the shortcomings by modifying the exosomes. Such exosomes derived from modified cells hold potential as future therapeutic strategies.
Keywords: Osteoarthritis; Yes-associated protein; exosomes; extracellular matrix; miR-140-5p.; synovial mesenchymal stem cells.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures



References
-
- Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O. et al. A Randomized, Controlled Trial of Total Knee Replacement. The New England journal of medicine. 2015;373:1597–606. - PubMed
-
- Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H. et al. Osteoarthritis. Lancet (London, England) 2015;386:376–87. - PubMed
-
- Agricola R, Heijboer MP, Roze RH, Reijman M, Bierma-Zeinstra SM, Verhaar JA. et al. Pincer deformity does not lead to osteoarthritis of the hip whereas acetabular dysplasia does: acetabular coverage and development of osteoarthritis in a nationwide prospective cohort study (CHECK) Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:1514–21. - PubMed
-
- Dezateux C, Rosendahl K. Developmental dysplasia of the hip. Lancet (London, England) 2007;369:1541–52. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

