Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor
- PMID: 28042336
- PMCID: PMC5197066
- DOI: 10.7150/thno.17155
Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor
Abstract
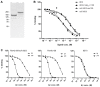
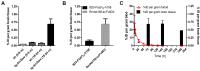
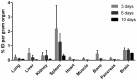
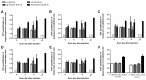
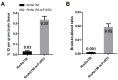
The blood-brain barrier (BBB) is an obstacle for antibody passage into the brain, impeding the development of immunotherapy and antibody-based diagnostics for brain disorders. In the present study, we have developed a brain shuttle for active transport of antibodies across the BBB by receptor-mediated transcytosis. We have thus recombinantly fused two single-chain variable fragments (scFv) of the transferrin receptor (TfR) antibody 8D3 to the light chains of mAb158, an antibody selectively binding to Aβ protofibrils, which are involved in the pathogenesis of Alzheimer's disease (AD). Despite the two TfR binders, a monovalent interaction with TfR was achieved due to the short linkers that sterically hinder bivalent binding to the TfR dimer. The design enabled efficient receptor-mediated brain uptake of the fusion protein. Two hours after administration, brain concentrations were 2-3% of the injected dose per gram brain, comparable to small molecular drugs and 80-fold higher than unmodified mAb158. After three days, fusion protein concentrations in AD transgenic mouse brains were 9-fold higher than in wild type mice, demonstrating high in vivo specificity. Thus, our innovative recombinant design markedly increases mAb158 brain uptake, which makes it a strong candidate for improved Aβ immunotherapy and as a PET radioligand for early diagnosis and evaluation of treatment effect in AD. Moreover, this approach could be applied to any target within the brain.
Keywords: BBB shuttle /TfR /antibodies / Alzheimer's disease/ immunotherapy/PET..
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures



References
-
- Bard F, Cannon C, Barbour R, Burke R-L, Games D, Grajeda H. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
-
- Pardridge WM. Blood-brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opin Drug Deliv. 2015;12:207–222. - PubMed
-
- Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y. et al. Boosting Brain Uptake of a Therapeutic Antibody by Reducing Its Affinity for a Transcytosis Target. Sci Transl Med. 2011;3:84ra44–84ra44. - PubMed
-
- Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P. et al. Increased Brain Penetration and Potency of a Therapeutic Antibody Using a Monovalent Molecular Shuttle. Neuron. 2014;81:49–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

