Effects of Mono-(2-Ethylhexyl) Phthalate and Di-(2-Ethylhexyl) Phthalate Administrations on Oocyte Meiotic Maturation, Apoptosis and Gene Quantification in Mouse Model
- PMID: 28042535
- PMCID: PMC5086329
- DOI: 10.22074/cellj.2016.4717
Effects of Mono-(2-Ethylhexyl) Phthalate and Di-(2-Ethylhexyl) Phthalate Administrations on Oocyte Meiotic Maturation, Apoptosis and Gene Quantification in Mouse Model
Abstract
Objective: Phthalates, which are commonly used to render plastics into soft and flexible materials, have also been determined as developmental and reproductive toxicants in human and animals. The purpose of this study was to evaluate the effect of mono-(2- ethylhexyl) phthalate (MEHP) and di-(2-ethylhexyl) phthalate (DEHP) oral administrations on maturation of mouse oocytes, apoptosis and gene transcription levels.
Materials and methods: In this experimental study, immature oocytes recovered from Naval Medical Research Institute (NMRI) mouse strain (6-8 weeks), were divided into seven different experimental and control groups. Control group oocytes were retrieved from mice that received only normal saline. The experimental groups I, II or III oocytes were retrieved from mice treated with 50, 100 or 200 µl DEHP (2.56 µM) solution, respectively. The experimental groups IV, V or VI oocytes were retrieved from mouse exposed to 50, 100 or 200 µl MEHP (2.56 µM) solution, respectively. Fertilization and embryonic development were carried out in OMM and T6 medium. Apoptosis was assessed by annexin V-FITC/Dead Cell Apoptosis Kit, with PI staining. In addition, the mRNA levels of Pou5f1, Ccna1 and Asah1 were examined in oocytes. Finally, mouse embryo at early blastocyst stage was stained with acridine-orange (AO) and ethidium-bromide (EB), in order to access their viability.
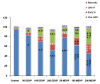
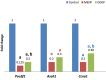
Results: The proportion of oocytes that progressed up to metaphase II (MII) and 2-cells embryo formation stage was significantly decreased by exposure to MEHP or DEHP, in a dose-dependent manner. Annexin V and PI positive oocytes showed greater quantity in the treated mice than control. Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) revealed that expression levels of Pou5f1, Asah1 and Ccna1 were significantly lower in the treated mouse oocytes than control. The total cell count for blastocyst developed from the treated mouse oocytes was lower than the controls.
Conclusion: These results indicate that oral administration of MEHP and DEHP could negatively affect mouse oocyte meiotic maturation and development in vivo, suggesting that phthalates could be risk factors for mammalians' reproductive health. Additionally, phthalate-induced changes in Pou5f1, Asah1 and Ccna1 transcription level could explain in part, the reduced developmental ability of mouse-treated oocytes.
Keywords: Apoptosis; Gene Expression; Oocyte Maturation.
Figures

References
-
- Bernanke J, Köhler HR. The impact of environmental chemicals on wildlife vertebrates. Rev Environ Contam Toxicol. 2009;198:1–47. - PubMed
-
- Kavlock R, Barr D, Boekelheide K, Breslin W, Breysse P, Chapin R, et al. NTP-CERHR expert panel update on the reproductive and developmental toxicity of di (2-ethylhexyl) phthalate. Reprod Toxicol. 2006;22(3):291–399. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
