Efficacy of carboplatin-based preoperative chemotherapy for triple-negative breast cancer. A meta-analysis of randomized controlled trials
- PMID: 28042625
- PMCID: PMC5278059
- DOI: 10.15537/smj.2017.1.14969
Efficacy of carboplatin-based preoperative chemotherapy for triple-negative breast cancer. A meta-analysis of randomized controlled trials
Abstract
Objectives: To evaluate the efficacy and safety of carboplatin-based preoperative chemotherapy in triple-negative breast cancer patients (TNBC).
Methods: PubMed, EMBASE, the Web of Science, the Cochrane Library, major clinical trial registries, and abstract collections from major international meetings were systematically searched for relevant randomized controlled trials. Endpoints included rates of pathologic complete response (pCR), overall response (ORR), breast-conserving surgery (BCS) and toxicity. Pooled relative risk (RR) was calculated for each endpoint using a fixed- or random-effect model depending on the heterogeneity among included studies.
Results: A total of 5 randomized controlled trials involving 1007 patients were included in the meta-analysis. Carboplatin-based chemotherapy was associated with a pooled pCR rate of 53.3%, which was significantly higher than the rate associated with non-carboplatin therapy (37.8%, RR: 1.41, 95% confidence interval [CI]: 1.23 to 1.62, p less than 0.00001). Compared with non-carboplatin therapy (48.1%), carboplatin-based chemotherapy increased BCS rate (59.7%, RR: 1.24, 95%CI: 1.06 to 1.46, p=0.007). Carboplatin-based chemotherapy was associated with similar ORR as non-carboplatin therapy. Carboplatin-based chemotherapy was associated with higher incidence of grade 3 or 4 anemia, neutropenia, febrile neutropenia, and thrombocytopenia than non-carboplatin therapy, while the 2 regimens were associated with similar incidence of fatigue, leucopenia, and nausea/vomiting.
Conclusion: The available evidence suggests that carboplatin-based preoperative chemotherapy is associated with significantly better pCR and BCS rates than non-carboplatin-based therapy in TNBC patients.
Figures

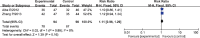
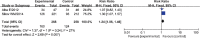
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. - PubMed
-
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. - PubMed
-
- Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer--current status and future directions. Ann Oncol. 2009;20:1913–1927. - PubMed
-
- Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

